Description
Product Information
| Product Name | Cat.No. | Spec. |
| High Purity Plasmid DNA Miniprep Kit | G3630-100T | 100T |
Description/Introduction
This High Purity Plasmid DNA Miniprep Kit is designed for rapid and cost-effectives small-scale preparation of high quality plasmid DNA from recombinant E.coli cultures. This kit is based on modified SDS-alkali lysis method and utilizes an exclusive silica-based membrane technology in the form of a convenient spin column. Each Bind DNA Mini Column can recover up to 25 μg of plasmid DNA. The actual plasmid yield depend on the plasmid copy number and culture density. The purified plasmid DNA van be used in all molecular biology procedures such as conventional digestion with restriction enzymes, PCR, transformation and DNA sequencing.
Storage and Handling Conditions
High Purity Plasmid DNA Miniprep Kit can be stored at room temperature for up to 12 months. For longer storage, it is recommended to keep the kit at 4℃. after addition of RNase A, the resuspension solution Buffer S1 (Red) should be stored at 4℃.
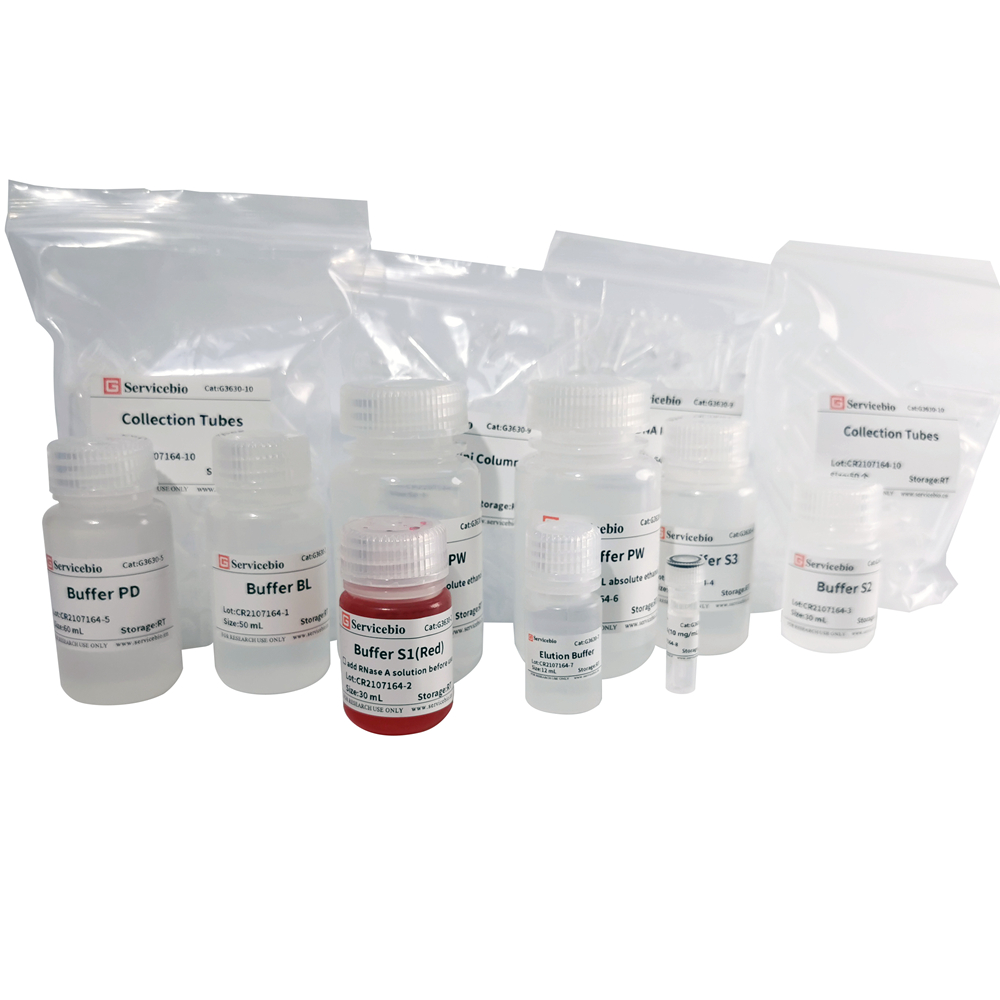
Component
| Component Number | Component | G3630-100T |
| G3630-1 | Buffer BL | 50 mL |
| G3630-2 | Buffer S1 (Red) | 30 mL |
| G3630-3 | Buffer S2 | 30 mL |
| G3630-4 | Buffer S3 | 40 mL |
| G3630-5 | Buffer PD | 60 mL |
| G3630-6 | Buffer PW | 2×15 mL |
| G3630-7 | Elution Buffer | 12 mL |
| G3630-8 | RNase A | 300 μL |
| G3630-9 | Bind DNA Mini Column | 100 |
| G3630-10 | Collection Tube | 100 |
| Product Manual | ||
Before starting (please read carefully)
1. If precipitate forms in the buffers during storage, it should be redissolved by incubating the buffers at 37℃, then cool back down to 25℃ before use. Do not shake the Buffer S2 too vigorously.
2. Add the provided RNase A solution to Buffer S1 (Red) and mix. The resuspension solution should be stored at 4℃.
3. Add 60 mL 100% ethanol to the Buffer PW and mark the bottle on the label.
4. (Optional step) Column balance: Put the Bind DNA Mini Columns into the Collection Tubes, add 500 μL solution Buffer BL to the adsorption column, centrifuge at 10,000 g for 1 min, remove the waste liquid in the Collection Tubes. Return the Bind DNA Mini Columns to the collection tube (please use the column treated on the day). In the column balancing step, the addition of Buffer BL can improve the adsorption capacity, homogeneity and stability of the Bind DNA Mini Columns, and eliminate the influence of high temperature, humidity or other adverse environmental factors on the Bind DNA Mini Columns.
Assay Protocol / Procedures
1. Bacterial culture collection: harvest bacterial culture from 1-5 mL of fresh bacterial culture incubated for 12-16 h by centrifugation at 8000-10000 rpm for 2 min at room temperature. Decant the supernatant and remove all remaining medium.
Note: For high-copy-number plasmids (such as pUc vectors, pBluescript vectors, pGEM vectors, pTZ vectors, pJET vectors), do not process more than 5 mL of bacterial culture. If more than 5 mL, the Bind DNA Mini Column capacity (25 μg) will be exceed and no increase in plasmid yield will be obtained. On the other hand, for low-copy-number plasmids (such as pBR322 and derivatives, pACYC and derivatives) and very low-copy plasmids (pSC101 and derivatives), it may be necessary to process larger volumes of bacterial culture (up to 10 mL) to recover a sufficient quantity of DNA.
2. Resuspend the pelleted cells in 250 µL of Buffer S1 (please ensure RNase A has been added before using). The bacteria should be resuspended completely by vortexing or pipetting up and down until no bacterial clumps remain. Let the uniform red bacterial suspension stand at room temperature for 5 min (Note: If bacterial clumps are incomplete mixed well, it will affect the lysis, resulting in low extraction amount and purity).
3. Lysis: add 250 µL of the Buffer S2 and mix thoroughly by inverting the tube 6-8 times until the solution becomes viscous and slightly clear. (Note: mix well gently, do not shake violently; At this time, the strain of bacteria liquid becomes clear and viscous. If it does not become clear, it may be due to too many bacteria and incomplete lysis, so the volume of bacteria should be reduced. After Buffer S2 is added and thoroughly mixed, the color of the solution is clear purple. If the purple is mixed with turbid red, it indicates that the cracking is not sufficient. Continue mixing until the color of the solution is completely clear purple. Do not incubate for more than 5 min to avoid denaturation of supercoiled plasmid DNA);
4. Neutralization: add 350 µL of the Buffer S3 and mix immediately and thoroughly by inverting the tube 6-8 times. At this time, the neutralized bacterial lysate should become cloud. After centrifugation at 13,000 g for 10-15 min (Note: Buffer S3 should be mixed immediately after adding, and a small amount of tiny white precipitate in the supernatant has no effect on subsequent experiments; There was a large amount of tiny white precipitate in the supernatant, and the supernatant could be taken after centrifugation again. After adding Buffer S3 and thoroughly mixing, the solution is clear yellow. If there is purple mixed in the yellow, it indicates that the renaturation is not sufficient. Continue to mix until the solution is completely clear yellow.), the collected supernatant was transferred to the Bind DNA Mini Columns, taking care not to pipette precipitation as much as possible. After centrifugation again at 10,000 g for 1 min, the waste liquid was discarded and the Bind DNA Mini Columns was put into the collection tubes.
5. Add 500 µL of the Buffer PD to the Bind DNA Mini Columns. Centrifuge for 1 min and discard the flow-through. Place the column back into the same collection tube.
6. Add 700 µL of the Buffer PW (Please check whether anhydrous ethanol has been added before using) to the Bind DNA Mini Columns. Centrifuge for 1 min and discard the flow-through. Place the Bind DNA Mini Columns back into the same collection tube. (Note: If the recovered DNA is used for salt-sensitive experiments, such as flat end joining experiment or direct sequencing, it is recommended that Buffer PW be added and let stand for 2-5 min before centrifugation.)
7. Repeat the wash procedure (step 7).
8. The Bind DNA Mini Columns was put into the collection tube and centrifuged at 10,000 g for 2 min to remove the residual liquid as far as possible. The Bind DNA Mini Columns was placed at room temperature for 5 min and dried thoroughly (Note: residual ethanol in rinse solution will affect subsequent enzyme digestion and PCR experiments).
9. Transfer the Bind DNA Mini Columns into a fresh 1.5 mL microcentrifuge tube. Add 50~100 μL of the Elution Buffer or ddH2O to the center of Bind DNA Mini Columns membrane to elute the plasmid DNA(It is better to pre-heat Elution Buffer or ddH2O at 60-65℃ before using). Take care not to contact the membrane with the pipette tip. Incubate for 2 min at room temperature and centrifuge for 2 min to collect DNA solution (Note: The volume of eluent should not be less than 50 μL, otherwise it will affect the efficiency of recovery. In order to improve the amount of DNA recovery, the centrifuged solution can be added to the Bind DNA Mini Columns again, placed at room temperature for 2 min, centrifuged at 10,000 g for 2 min, and the DNA solution is collected into the centrifugal tube. The pH value of the eluent has great influence on the elution efficiency. For subsequent sequencing, it is recommended to use ddH2O as eluent, and ensure that its pH value is within the range of 7.0-8.5. If pH value is lower than 7.0, elution efficiency will be reduced. DNA products should be stored at -20℃ to prevent DNA degradation).
Note:
1. The amount of plasmid extracted was related to the bacterial culture concentration and plasmid copy number. If the plasmid is low copy plasmid or large plasmid larger than 10 KB, the number of bacteria should be increased (5-10 mL for overnight bacterial culture) and the amount of Buffer S1, S2 and S3 should be increased in proportion. The Elution Buffer should be pre-heated in a 60-65℃ water bath, which can be appropriately extended during adsorption and Elution to extract efficiency. The other steps are the same.
2. Please tighten the lid in time to prevent solvent volatilization after each solution is used.
3. Please wear experimental suits and disposable gloves when operation.
For Research Use Only!
Ver. No.: V1.0-202102
|
Cat.No.
|
Product Name
|
Spec.
|
Operation
|
|---|
|
G3630-100T
|
High Purity Plasmid DNA Miniprep Kit
|
100 T
|
|
|
G3631-100T
|
Universal DNA Purification and Gel Extraction Kit
|
100 T
|
|
|
G3632-50T
|
Bacterial Genomic DNA Extraction Kit
|
50 T
|
|
|
G3633-50T
|
Tissue/Cell/Blood Genomic DNA Extraction Kit
|
50 T
|