Description
| Product Description | Related Products | Message Inquiry | Notes | Download Instructions |
|---|---|---|---|---|
| Product Information | ||||
| Product Name | Plant Genomic DNA Extraction Kit | |||
| Product Number | G3634-50T | |||
| Specification | 50T |
Product Introduction
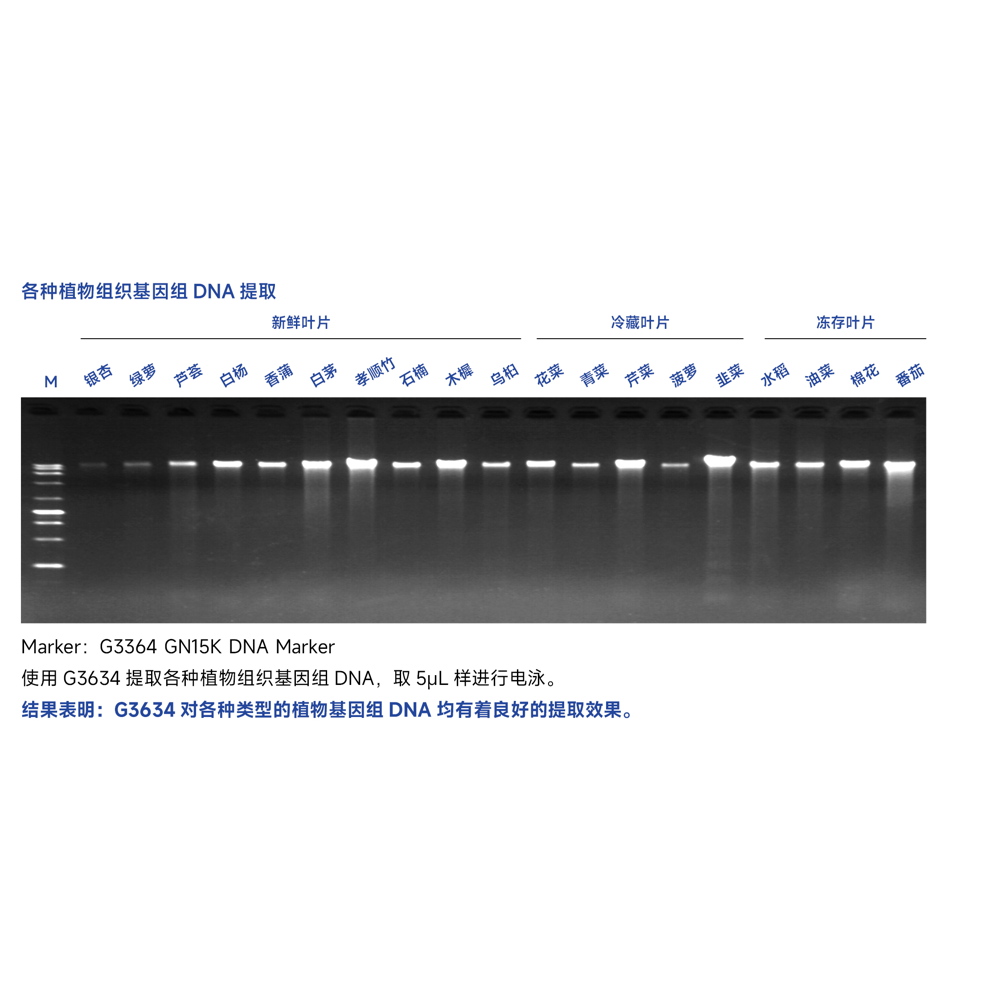
This kit utilizes centrifugal adsorption column technology and a unique lysis buffer system to safely, quickly, and efficiently extract genomic DNA from various simple plant tissues. After complete grinding of the plant samples with liquid nitrogen and thorough mixing with the lysis solution, genomic DNA is rapidly released, and the extraction process is completed within 1 hour. This kit can extract purified high-purity genomic DNA ranging from 50-100 mg of plant tissue, yielding 1-10 µg. The extracted genomic DNA can be used in various conventional molecular biology experiments such as PCR amplification, enzyme digestion reactions, Southern hybridization, as well as RAPD, AFLP, RFLP, and other applications.
Storage and Transportation
RNase A is transported on wet ice and stored at -20°C; other reagents are transported and stored at room temperature. The kit has a shelf life of 12 months.
Composition
[Note: The composition section is missing in the provided text.]
Please note that the translation provided is based on the information provided and may not be an exact translation.
| Component Number | Component | G3634–50T |
| G3634-1 | Buffer PGL1 | 25 mL |
| G3634-2 | Buffer PGL2 | 7 mL |
| G3634-3 | Buffer PD | 9 mL |
| G3634-4 | Buffer PW | 24 mL |
| G3634-5 | RNase A | 350 μL |
| G3634-6 | DNA Spin Columns | 50个 |
| G3634-7 | Collection tubes | 50个 |
| G3634-8 | Buffer TE | 15 mL |
| instruction | 1 |
Instructions for Use (Please read carefully)
- Avoid repeated freezing and thawing of plant samples stored at ultra-low temperatures, as it can result in decreased quality and yield of extracted DNA.
- If any precipitation is observed in Buffer PGL1, heat it at 65°C until the precipitation disappears and mix well before use.
- Before use, add the specified amount (as indicated on the bottle) of ethanol to Buffer PD and Buffer PW.
Operating Steps
- Plant tissue sample lysis:
a. (Recommended) Take fresh or ultra-low temperature frozen plant tissue of 50-100 mg and quickly transfer it to a 2.0 mL RNase-Free grinding tube (recommended HT-200-M) containing 2-3 3 mm grinding beads (recommended G0203-150G) pre-cooled with liquid nitrogen. Place the grinding tube on a homogenizer (recommended KZ-5F-3D) (pre-cool the adapter in liquid nitrogen before placing the grinding tube). Grind until the sample turns into a powdered form (incomplete grinding will affect DNA yield and quality). Then add 400 μL Buffer PGL1 and 6 μL RNase A. Use a pipette to mix until there is no visible precipitation in the lysis solution. Let it sit at room temperature for 10 minutes.
b. Take fresh or ultra-low temperature frozen plant tissue of 50-100 mg and quickly transfer it to a pre-cooled 1.5 mL RNase-free centrifuge tube. Add liquid nitrogen and grind the tissue with a pestle, continuously adding liquid nitrogen until the sample is ground into a powdered form (incomplete grinding will affect DNA yield and quality). Then add 400 μL Buffer PGL1 and 6 μL RNase A. Use a pipette to mix until there is no visible precipitation in the lysis solution. Let it sit at room temperature for 10 minutes.
c. Take fresh or ultra-low temperature frozen plant tissue of 50-100 mg and quickly transfer it to a pre-cooled mortar with liquid nitrogen. Add liquid nitrogen and grind the tissue with a pestle, continuously adding liquid nitrogen until the sample is ground into a powdered form (incomplete grinding will affect DNA yield and quality). Transfer the powdered sample to a 1.5 mL RNase-free centrifuge tube containing 400 μL Buffer PGL1 and 6 μL RNase A. Use a pipette to mix until there is no visible precipitation in the lysis solution. Let it sit at room temperature for 10 minutes.
- Add 130 μL Buffer PGL2 to the centrifuge tube, mix well by pipetting, and place it on ice for 5 minutes.
- Centrifuge at 12,000 rpm for 5 minutes and transfer the supernatant to a new centrifuge tube.
- Add Buffer PD to the centrifuge tube in an equal volume to the supernatant. Invert the tube several times to mix.
- Place the DNA Spin Column on the Collection Tube and transfer the mixture to the DNA Spin Column. Do not exceed 600 µL per addition; if it exceeds 600 µL, add in batches.
- Centrifuge at 12,000 rpm for 30 seconds and discard the filtrate. Place the DNA Spin Column back into the Collection Tube.
- Add 600 μL Buffer PW to the DNA Spin Column (adding Buffer PW along the tube wall helps to wash off residual salts on the tube wall). Centrifuge at 12,000 rpm for 30 seconds and discard the waste liquid.
- Repeat step 7.
- Place the DNA Spin Column back into the Collection Tube and centric
- Transfer the DNA Spin Column to a new 1.5 mL centrifuge tube and leave it at room temperature for 3-5 minutes to allow complete evaporation of residual ethanol from the DNA Spin Column.
- Add 50-100 μL of Buffer TE or Nuclease-free Water to the center of the DNA Spin Column membrane. Leave it at room temperature for 5 minutes (heating Buffer TE or Nuclease-free Water to 65°C can improve elution efficiency).
- Centrifuge at 12,000 rpm for 2 minutes to collect the DNA. To obtain a higher concentration of DNA, the eluate from the first elution can be added back to the DNA Spin Column, left at room temperature for 5 minutes, centrifuged at 12,000 rpm for 2 minutes, and the DNA collected again.
Precautions
- Use fresh plant tissue whenever possible to ensure the yield and integrity of genomic DNA.
- If the extracted DNA needs to be stored for a long time, elute it in Buffer TE and store at -80°C.
- Avoid repeated freezing and thawing of the obtained genomic DNA.
Appendix:
The extraction yield of genomic DNA from various plant samples using this kit is shown in the table below. The yield of genomic DNA depends on the plant species, tissue type, and growth stage. The recommended sample amount is indicated in the table below.
| Sample Name | Sample Amount | DNA Yield |
|---|---|---|
| Rice Leaf | 50 mg | 5-10 μg |
| Canola Leaf | 50 mg | 4-8 μg |
| Arabidopsis Leaf | 80 mg | 0.5-1 μg |
| Tobacco Leaf | 50 mg | 2-4 μg |
| Tomato Leaf | 50 mg | 2-5 μg |
| Leek Leaf | 50 mg | 3-6 μg |
| Celery Leaf | 50 mg | 2-4 μg |
| Pothos | 50 mg | 0.5-1 μg |
| Poplar | 50 mg | 1-2 μg |
| Chinese Cabbage | 50 mg | 1-2 μg |
Please note that the values provided for DNA yield are approximate ranges.