5. Paraffin-embedded (FFPE), formalin-fixed, tissues Tissue Genomic DNA Extraction Kit, 50 T $500,
Description
Product Information
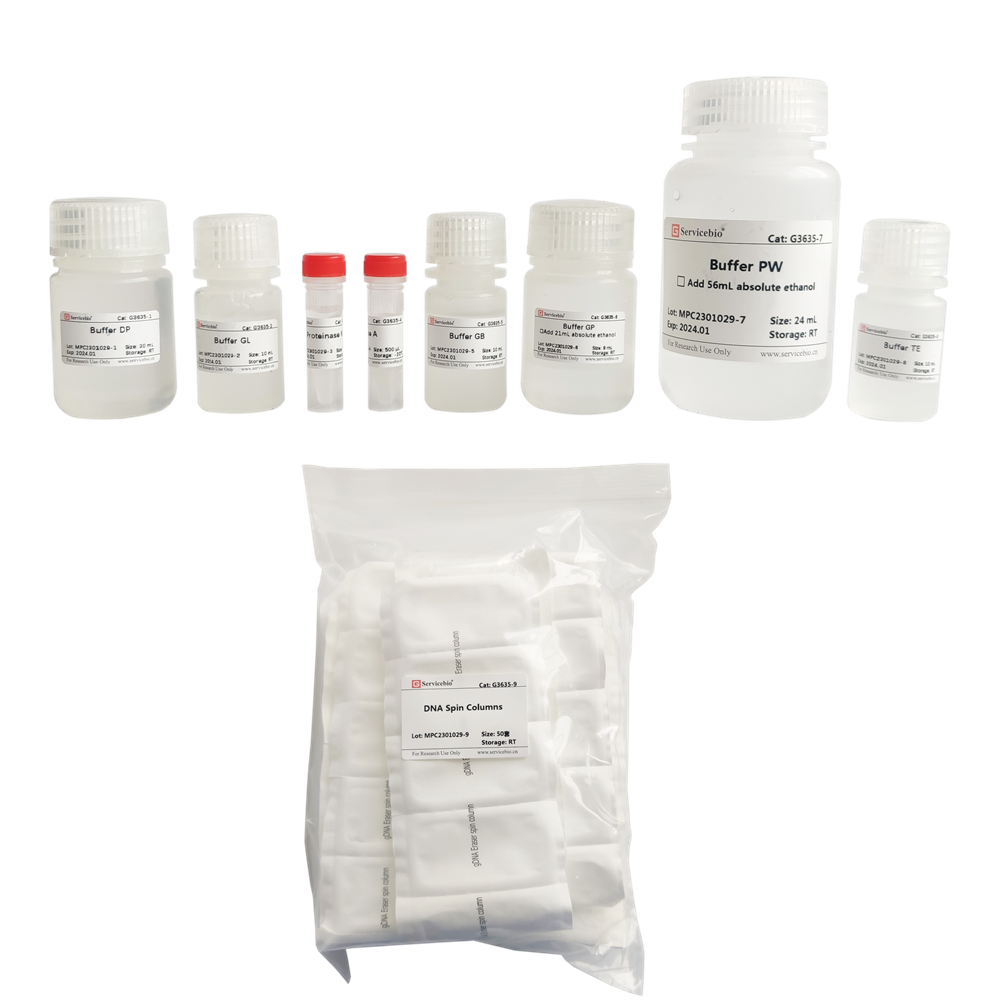
Product Name: Paraffin-embedded Tissue Genomic DNA Extraction Kit Product Code: G3635-50T Size: 50T
Product Description: This kit is designed for the extraction of genomic DNA from formalin-fixed, paraffin-embedded (FFPE) tissues. It utilizes a special dewaxing reagent that does not contain organic solvents such as xylene, making it safe, non-toxic, and effective in removing paraffin and releasing tissue samples. The kit employs a unique lysis buffer in combination with a column-based centrifugation method for rapid and efficient extraction of genomic DNA from paraffin-embedded tissues. The extraction process can be completed within 1 hour, yielding high DNA quantity and purity. It is suitable for various downstream applications, including PCR, Real-Time PCR, SNP genotyping, and other molecular biology experiments.
Storage and Transportation: RNase A and Proteinase K should be transported with wet ice (-20°C storage). Other reagents can be transported and stored at room temperature. The kit has a shelf life of 12 months.
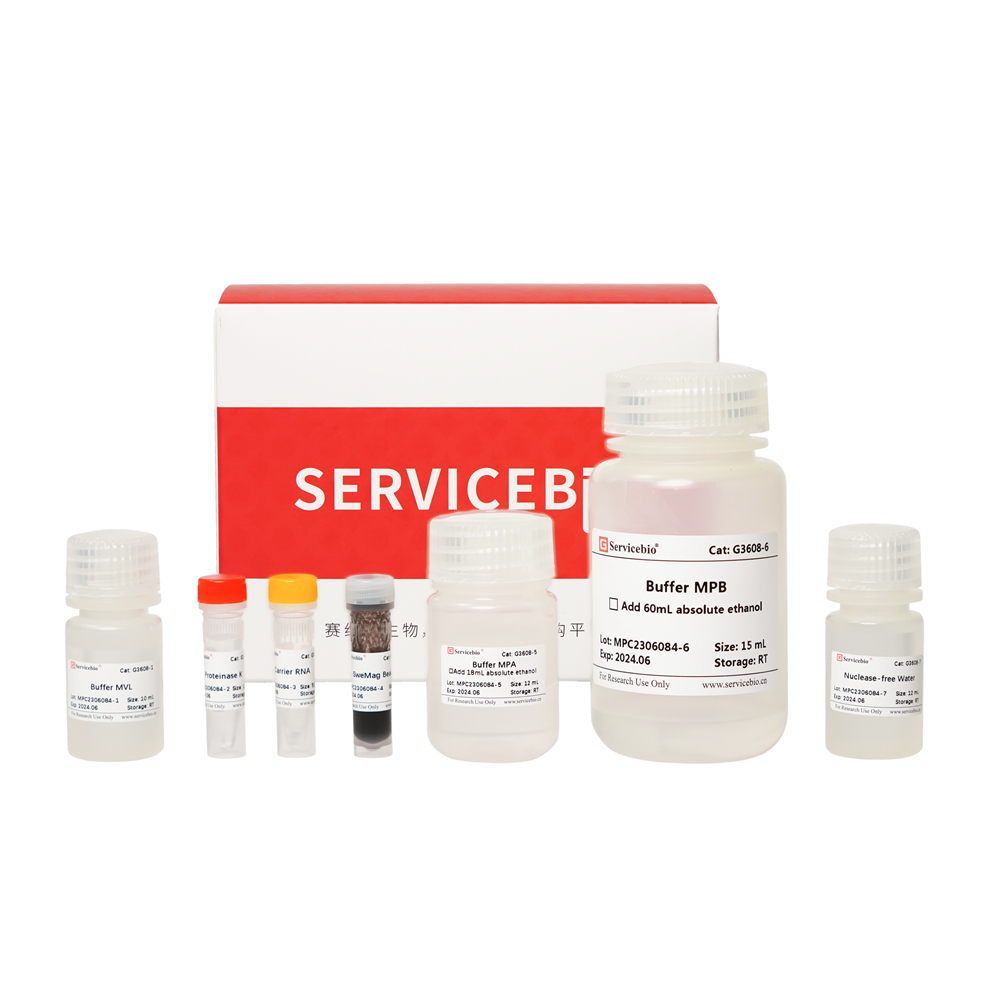
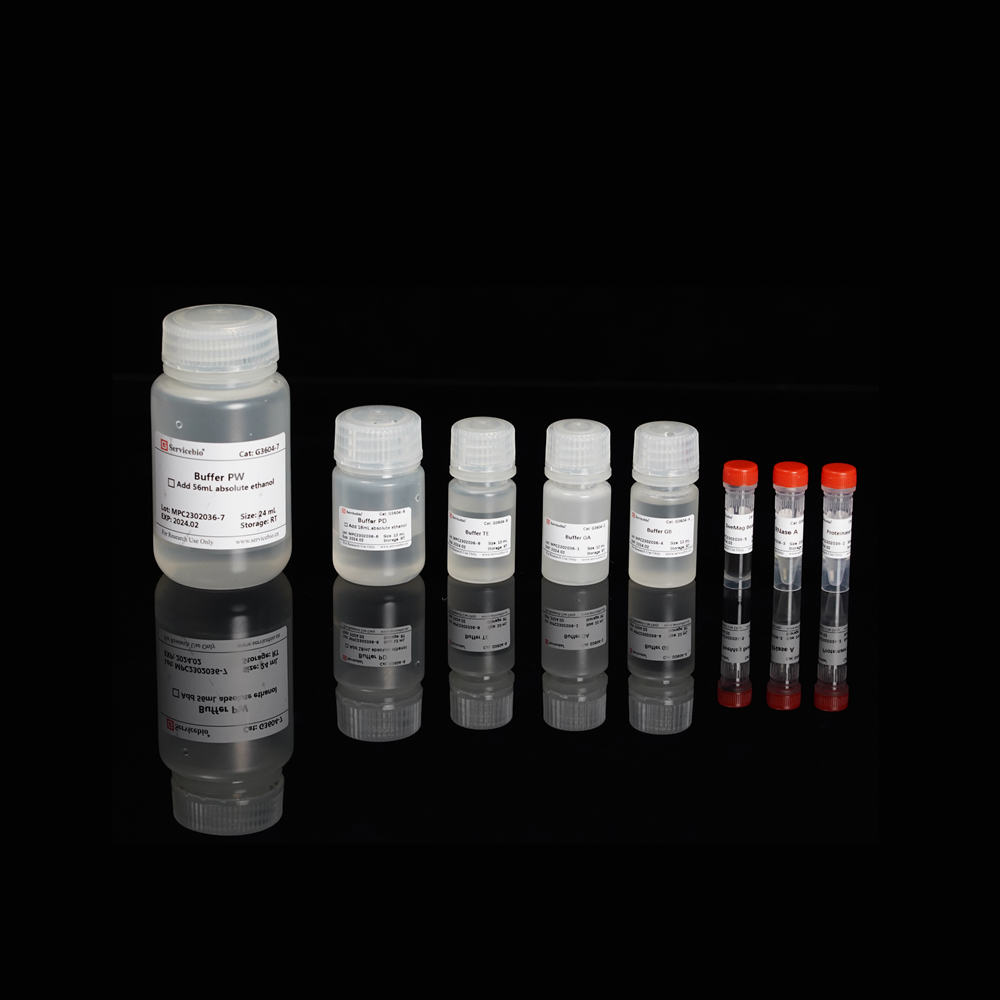
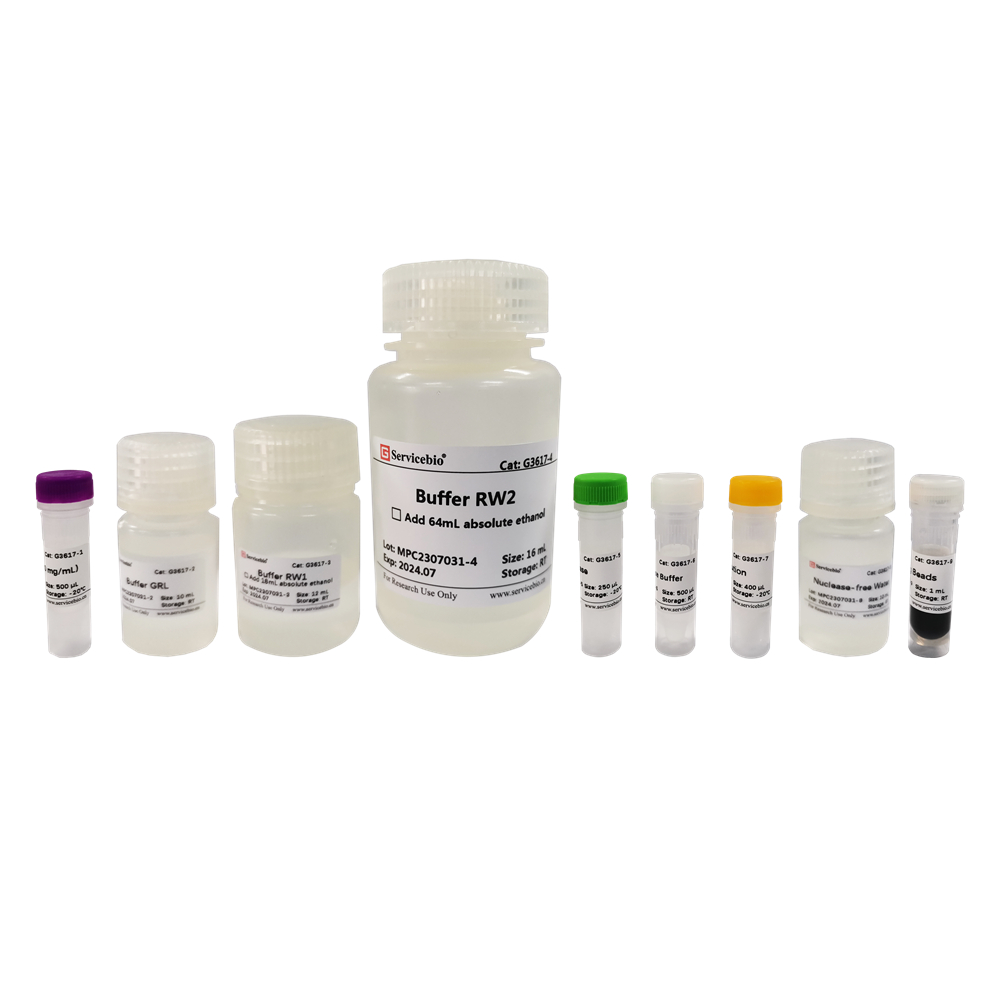
| Component Number | Component | G3635–50T |
| G3635-1 | Buffer DP | 30 mL |
| G3635-2 | Buffer GL | 10 mL |
| G3635-3 | Proteinase K | 1 mL |
| G3635-4 | RNase A | 500 μL |
| G3635-5 | Buffer GB | 10 mL |
| G3635-6 | Buffer GP | 9 mL |
| G3635-7 | Buffer PW | 24 mL |
| G3635-8 | Buffer TE | 10 mL |
| G3635-9 | DNA Spin Columns | 50 sets |
| User manual | 1 | |
Precautions before Use (Please read carefully):
- Prepare water baths or metal baths at 80°C, 56°C, and 90°C in advance.
- If Buffer GL and Buffer GB have precipitates, heat them at 65°C to dissolve. Use them after they return to room temperature.
- Before use, add 21 mL of absolute ethanol to Buffer GP and 56 mL of absolute ethanol to Buffer PW. Mix well before use.
Operating Steps:
- Sample Preparation:Paraffin Sections: Take 5-8 paraffin sections (1×1 cm2 in size), scrape and collect tissue fragments using a sterile surgical blade, and place them in a 1.5 mL centrifuge tube.Paraffin-embedded Tissue Blocks: Scrape approximately 30 mg of tissue sample using a sterile surgical blade, remove excess paraffin as much as possible, and mince the sample.
Formalin-fixed Tissue: Remove the liquid on the surface of the tissue using filter paper, mince approximately 30 mg of the sample, place it in a 1.5 mL centrifuge tube, add 500 μL of PBS buffer (pH 7.4), vortex for 10 s, centrifuge at room temperature at 12,000 rpm for 1 min, discard the supernatant, and repeat this step three times.
- Transfer the sample to a 1.5 mL centrifuge tube, add 500 μL of Buffer DP, incubate at 80°C for 3 min, and vortex for 10 s while still hot.
- Add 200 μL of Buffer GL to the centrifuge tube, vortex to mix, centrifuge at room temperature at 12,000 rpm for 1 min. The solution will form two layers (an upper oily phase and a lower aqueous phase).
- Add 20 μL of Proteinase K and 10 μL of RNase A to the lower aqueous phase. Gently pipette mix (try not to disrupt the layers), and incubate at 56°C for 20 min.
- Transfer the centrifuge tube to 90°C and incubate for 10 min (if there are substantial undigested tissue blocks, extend the incubation time to 20 min). Cool to room temperature.
- Add 200 μL of Buffer GB and 200 μL of absolute ethanol to the sample from the previous step. Vortex to mix.
- Centrifuge at room temperature at 12,000 rpm for 1 min. The solution will form two layers (an upper oily phase and a lower aqueous phase).
- Transfer the lower aqueous phase solution to a DNA Spin Column (avoid taking the upper oily phase solution and other impurities). Centrifuge at room temperature at 12,000 rpm for 2 min. Discard the filtrate.
- Add 500 μL of Buffer GP to the DNA Spin Column. Centrifuge at room temperature at 12,000 rpm for 1 min. Discard the filtrate.
- Add 600 μL of Buffer PW to the DNA Spin Column (pour the Buffer PW along the column wall to help flush out residual salts). Centrifuge at room temperature at 12,000 rpm for 1 min. Discard the filtrate.
- Repeat step 10.
- Place the DNA Spin Column on a collection tube. Centrifuge at room temperature at 12,000 rpm for 2 min. Remove the DNA Spin Column and place it in a new 1.5 mL centrifuge tube.
- Leave the DNA Spin Column uncapped at room temperature for 3-5