No products in the cart.
Description
- Product Name: Magnetic Bead-based High-Purity Plasmid DNA Large-Scale Extraction Kit
- Product Number: G3610-10T
- Specifications: 10T
Product Description:
This kit utilizes the reversible binding properties of highly superparamagnetic beads and a unique buffer system to rapidly and efficiently obtain 600-1500 μg of high-purity plasmid DNA from 100 mL of overnight bacterial culture. Plasmid DNA extracted using this kit can be used in molecular biology experiments such as restriction enzyme digestion, ligation reactions, PCR amplification, and sequencing.
Storage and Transportation:
- RNase A is transported on wet ice and should be stored at -20°C.
- Other reagents are transported at room temperature and can be stored at room temperature.
- Shelf life: 12 months.
-
Component Number Component G3610-10T Volume G3610-1 Buffer SP1 90 mL G3610-2 Buffer SP2 90 mL G3610-3 Buffer SP3 90 mL G3610-4 RNase A 180 μL G3610-5 Buffer SPD 110 mL G3610-6 Buffer PW 33 mL G3610-7 Buffer TE 30 mL G3610-8 SweMag Beads 7 mL -
Pre-Experiment Preparation
- Prepare a magnetic stand.
- Before use, add all the provided RNase A into Buffer SP1, and it can be stored at 4°C for up to 6 months.
- After using SP2, immediately close the cap to avoid prolonged exposure of the reagent to the air.
- Before using SP3, pre-cool it at 4°C.
- Before use, add 77 mL of anhydrous ethanol to Buffer PW.
Experimental Procedure
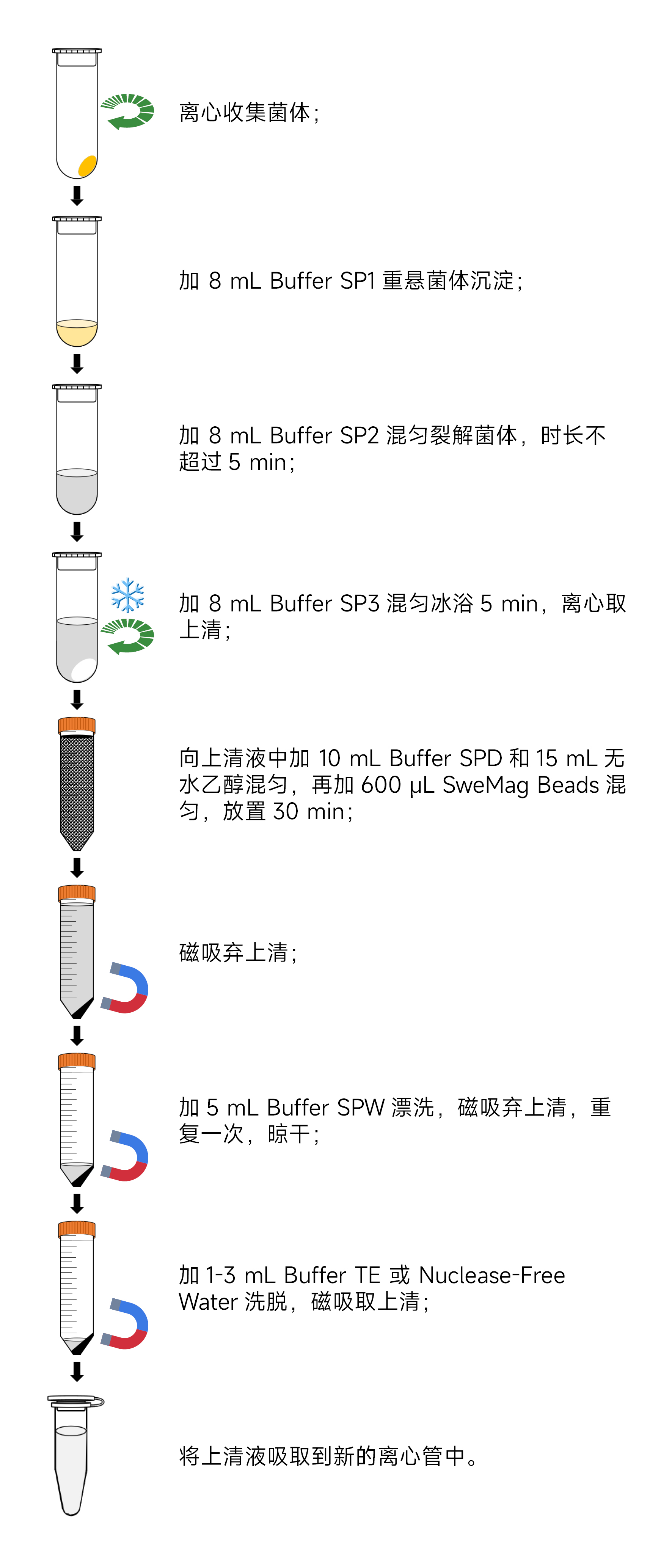
- Take 100 mL of overnight cultured bacterial liquid (for low-copy plasmids, it is recommended to take 200 mL of overnight cultured bacterial liquid). Centrifuge at 10,000 rpm (~11,500×g) for 3 minutes at room temperature to collect bacterial cells in a 50 mL centrifuge tube (try to remove all the supernatant).
- Add 8 mL of SP1 (ensure that RNase A has been added), and thoroughly suspend the bacterial cells using a pipette or vortex oscillator (ensure complete dispersion of bacterial cells to avoid affecting lysis, which could lead to lower quality and purity of the extracted plasmid).
- Add 8 mL of SP2 and immediately gently invert the tube 8-10 times to fully lyse the bacterial cells (do not shake vigorously, and ensure this step is completed within 5 minutes). At this point, the solution should become clear and viscous. If it does not become clear, there may be an excess of bacterial cells; invert a few more times until the solution turns clear.
- Add 8 mL of SP3 (pre-cooled to 4°C) and immediately gently invert the tube 10-12 times. The solution should form tight clumps. Place it on ice for 5 minutes, then centrifuge at 10,000 rpm at 4°C for 20 minutes. Collect the supernatant into a 50 mL Nuclease-free centrifuge tube (provided by the user).
- Add 10 mL of SPD and gently invert several times. Then, add 600 μL of SweMag Beads (vortex the SweMag Beads to ensure even dispersion) and gently invert several times until the beads are evenly dispersed. Allow it to stand at room temperature for 30 minutes, gently inverting every 3-5 minutes.
- Place the 50 mL centrifuge tube on a magnetic stand for 2 minutes. Afterward, gently invert the tube along with the magnetic stand several times to wash off the beads adhering to the tube cap. Continue to let it stand until the liquid is clear, then discard the liquid.
- Remove the tube from the magnetic stand and add 5 mL of SPW (check whether anhydrous ethanol has been added). Gently invert to ensure even dispersion of the beads. Then, place the tube on the magnetic stand and gently invert the tube along with the magnetic stand several times to wash off the beads adhering to the tube cap. Continue to let it stand until the liquid is clear, then discard the liquid.
- Repeat step 7.
- Open the tube cap and let it sit at room temperature for 5-10 minutes or at 65°C for 3-5 minutes to allow the ethanol to completely evaporate (avoid over-drying the beads to prevent affecting the nucleic acid yield).
- Remove the magnetic stand, add 1-3 mL of buffer TE or Nuclease-free Water, and gently pipette until the beads are evenly dispersed. Let it stand at room temperature for 3-5 minutes.
- Place the tube back on the magnetic stand until the beads are fully adsorbed. Aspirate the supernatant into a new Nuclease-free centrifuge tube to obtain high-concentration and high-purity plasmid DNA.
- Store the obtained plasmid DNA at -20°C for future use.
Precautions
- Before operation, please read this product manual carefully.
- Avoid freezing the magnetic bead suspension during storage.
- Magnetic beads tend to settle, so shake or vortex thoroughly before use.
- Ensure complete evaporation of ethanol before eluting plasmids to prevent residual ethanol from affecting downstream experiments.
- Do not overdry the magnetic beads for an extended period, as this may affect DNA elution efficiency.
- For long-term storage of plasmid DNA, it is recommended to elute with Buffer TE.
- For your safety and health, wear laboratory attire and disposable gloves during the operation.