Description
- Product Name: Magnetic Bead-based Viral DNA/RNA Extraction Kit
- Product Number: G3608-50T
- Specification: 50T
Product Description:
This kit is designed for the rapid separation of various viral DNA/RNA from serum, plasma, urine, cell culture supernatant, virus stocks, and virus-infected tissue. Using our independently developed superparamagnetic beads, nucleic acids specifically adsorb to the surface of magnetic particles under the action of a special buffer system. Proteins, cell debris, and other contaminants can be effectively washed away, and high-quality DNA/RNA can be eluted using Nuclease-free Water. The high-quality DNA/RNA obtained can be directly used for downstream molecular biology experiments such as PCR, cDNA synthesis, RT-qPCR, and more.
Storage and Transportation:
Proteinase K and Carrier RNA are transported on wet ice and should be stored at -20°C. The rest of the reagents are transported at room temperature and should be stored at room temperature. The shelf life is 12 months.
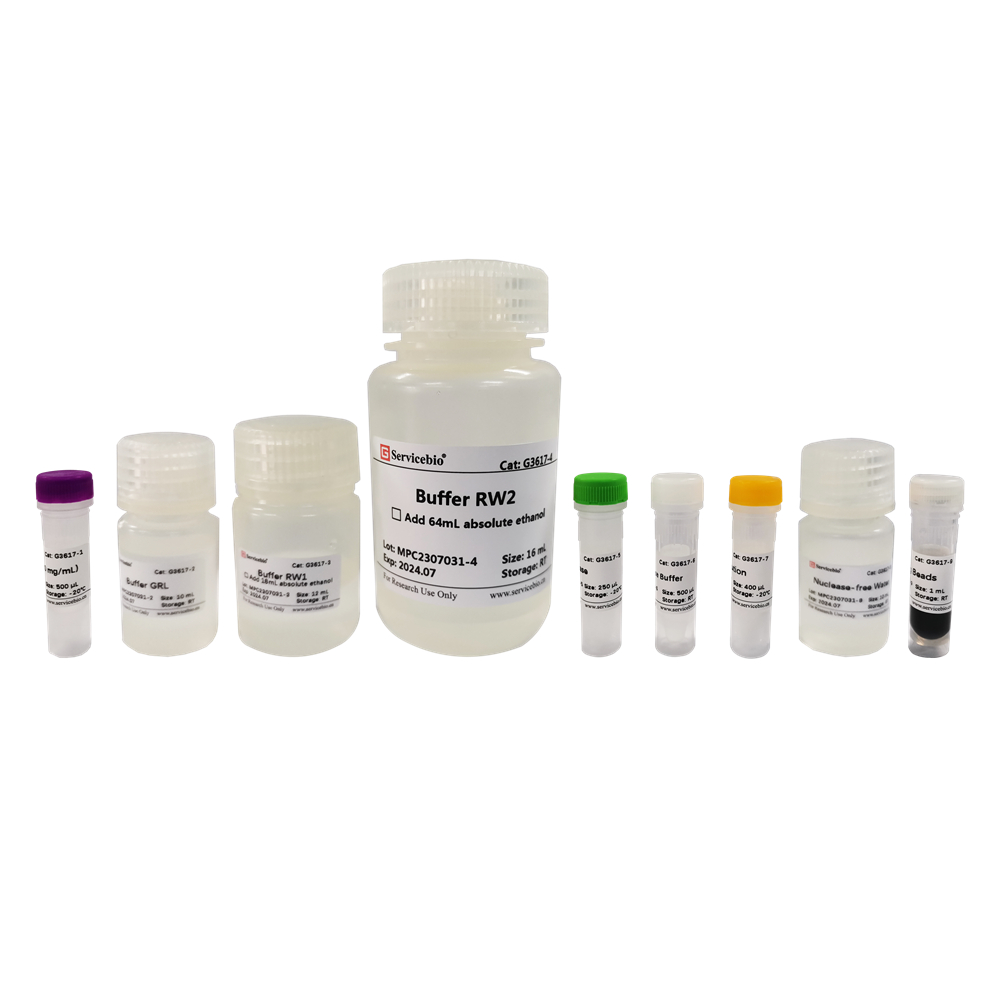
Here is the component information for the Magnetic Bead-based Viral DNA/RNA Extraction Kit in tabular format:
| Component Number | Component | G3608-50T |
|---|---|---|
| G3608-1 | Buffer MVL | 10 mL |
| G3608-2 | Proteinase K | 1 mL |
| G3608-3 | Carrier RNA | 50 μL |
| G3608-4 | SweMag Beads | 1 mL |
| G3608-5 | Buffer MPA | 12 mL |
| G3608-6 | Buffer MPB | 15 mL |
| G3608-7 | Nuclease-free Water | 12 mL |
Additionally, there is 1 copy of the instruction manual included.
Here is the translated version of the procedure for the Magnetic Bead-based Viral DNA/RNA Extraction Kit:
Preparation Before Use
- If Buffer MVL and Buffer MPA have precipitated, heat them to 37°C until dissolved. Allow them to return to room temperature before use.
- For the first-time use, add 18 mL of ethanol to Buffer MPA and 60 mL of ethanol to Buffer MPB.
- For the first-time use, aliquot the Carrier RNA and store it at -20°C to avoid repeated freeze-thaw cycles.
- Pre-cool the tissue homogenizer before use.
- Prepare a magnetic rack.
Experimental Procedure
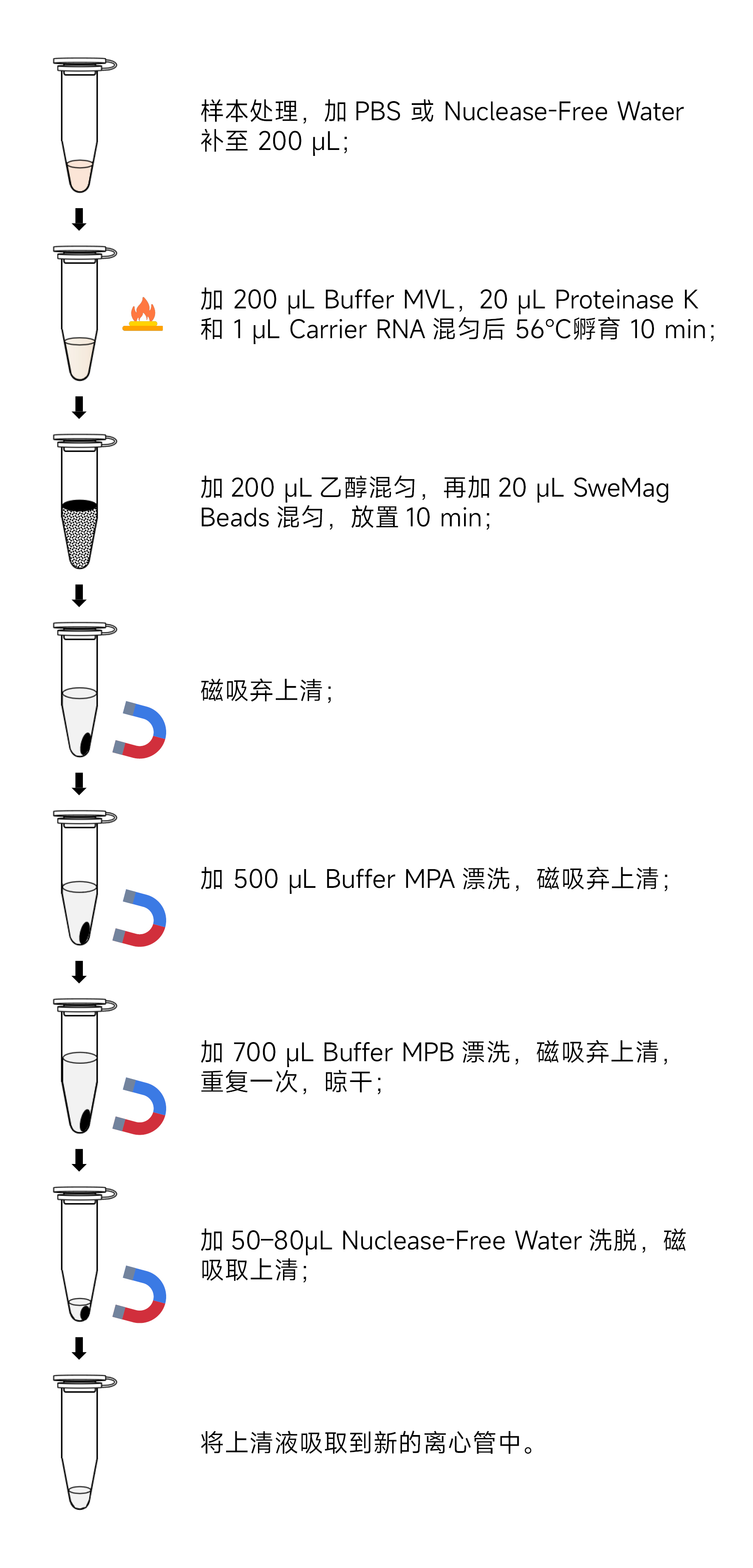
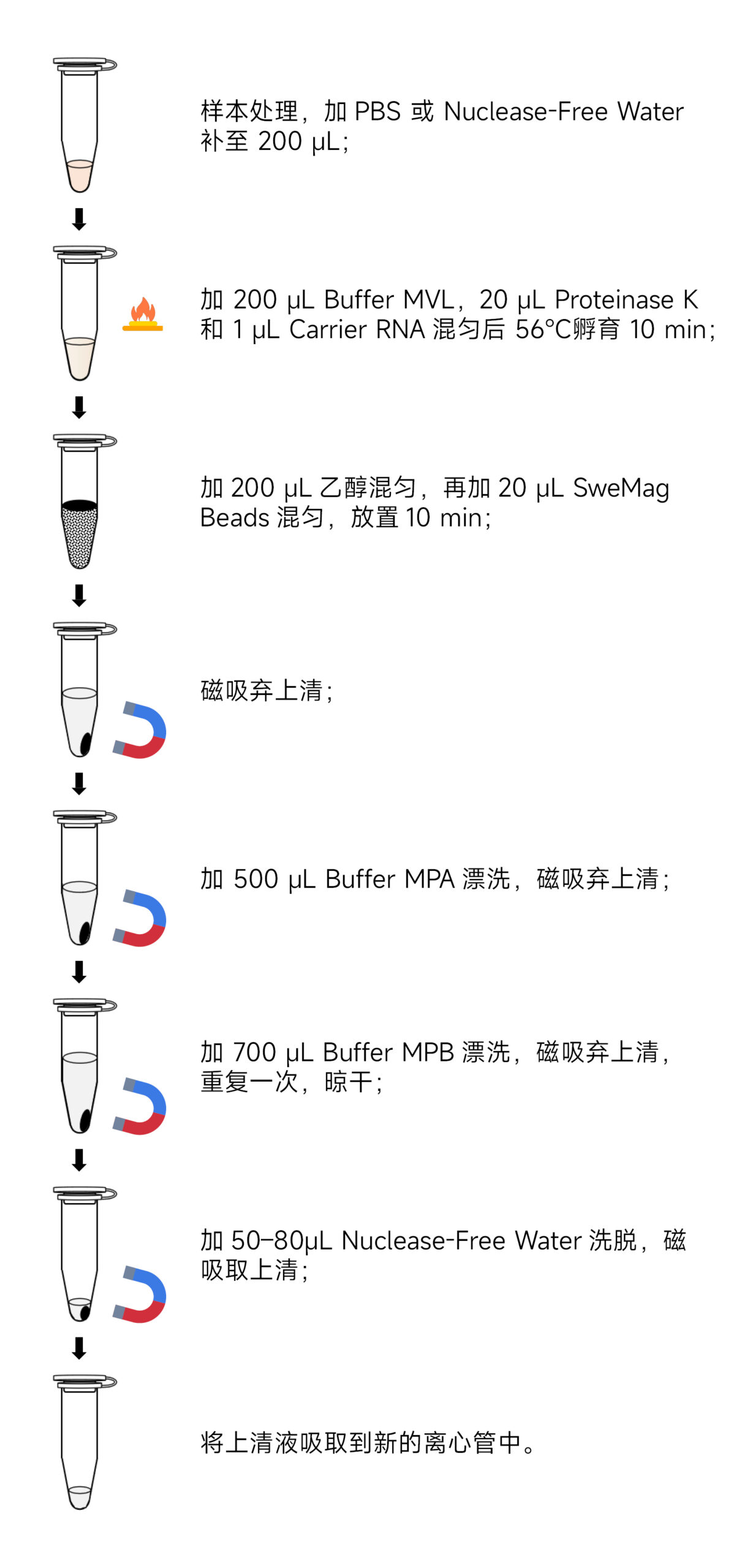
Processing of Virus Samples:
a) Lysis of serum, plasma, urine, cell culture supernatant, and virus stock: Take 10-200 μL of serum, plasma, urine, cell culture supernatant, or virus stock. If the initial volume is less than 200 μL, you can top up with PBS or Nuclease-free Water to reach 200 μL.
b) Lysis of infected virus tissue: Take 10-20 mg of fresh or ultra-low-temperature frozen virus-infected tissue. Place it in a 1.5 mL Nuclease-free centrifuge tube or a dedicated homogenization tube containing 2-3 pieces of 3 mm grinding beads (recommended G0203). Immediately place the tube with tissue in liquid nitrogen. Then, use a tissue homogenizer (recommended KZ-5F-3D) to thoroughly homogenize the tissue at low temperatures until it becomes a uniform paste (if the tissue is not thoroughly homogenized, it will affect the yield and quality of DNA/RNA). After homogenization, add 200 μL of PBS or Nuclease-free Water.
- Add 200 μL of Buffer MVL, 20 μL of Proteinase K, and 1 μL of Carrier RNA. Mix well and incubate at 56°C for 10 minutes.
- Add 200 μL of ethanol, invert to mix, and then add 20 μL of SweMag Beads (vortex SweMag Beads before use until they are evenly dispersed). Invert several times until the beads are evenly dispersed.
- Allow the mixture to sit at room temperature for 10 minutes, gently mixing 2-3 times using a pipette or vortex mixer until the beads are evenly dispersed.
- Place the centrifuge tube on a magnetic rack for 30 seconds until the beads are fully adsorbed. Then, gently invert the tube several times along with the magnetic rack to wash off the beads that may be stuck to the tube walls. Discard the supernatant (do not aspirate the beads to avoid affecting the extraction efficiency).
- Remove the magnetic rack and add 500 μL of Buffer MPA. Use a pipette to gently mix until the beads are evenly dispersed. Place the centrifuge tube on the magnetic rack for 30 seconds, then gently invert the tube several times along with the magnetic rack to wash off the beads and salt that may be stuck to the tube walls. Discard the supernatant (be sure to aspirate all residual liquid in the centrifuge tube to avoid affecting the extraction efficiency).
- Remove the magnetic rack and add 700 μL of Buffer MPB. Use a pipette to gently mix until the beads are evenly dispersed. Place the centrifuge tube on the magnetic rack for 30 seconds, then gently invert the tube several times along with the magnetic rack to wash off the beads and salt that may be stuck to the tube walls. Discard the supernatant (be sure to aspirate all residual liquid in the centrifuge tube to avoid affecting the extraction efficiency).
- Repeat step 6.
- Remove the tube cap and let it sit at room temperature for 5-10 minutes or at 65°C for 3-5 minutes to allow ethanol to evaporate completely (to avoid over-drying the beads, which could affect nucleic acid yield).
- Remove the magnetic rack and add 50-80 μL of Nuclease-free Water. Use a pipette to gently mix until the beads are evenly dispersed. Allow to sit at room temperature for 3 minutes.
- Place the centrifuge tube on the magnetic rack until the beads are fully adsorbed. Aspirate the supernatant into a new Nuclease-free centrifuge tube to obtain high-purity DNA/RNA.