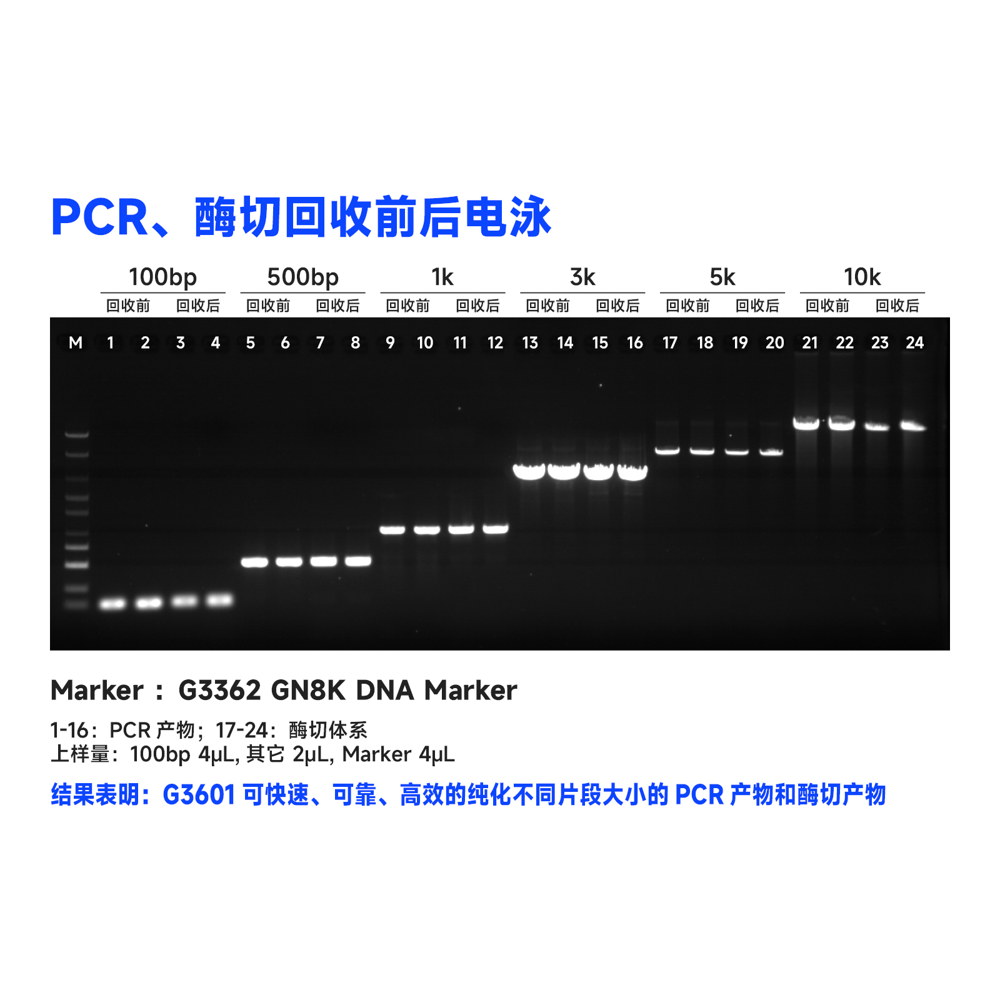
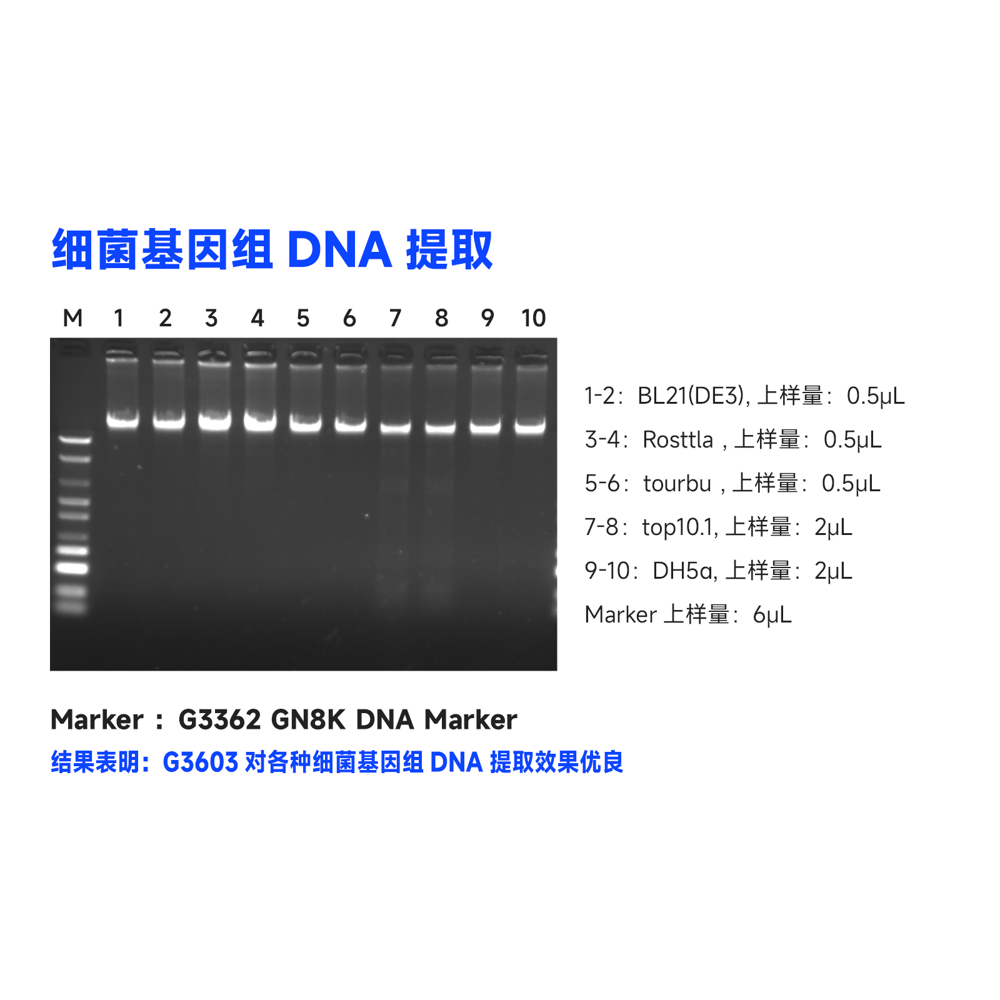
Description
Product Information
Product Name
Product Number
Specification
Magnetic Bead-Based Plasmid DNA Mini-Extraction Kit
G3600-100T
100T
Product Description
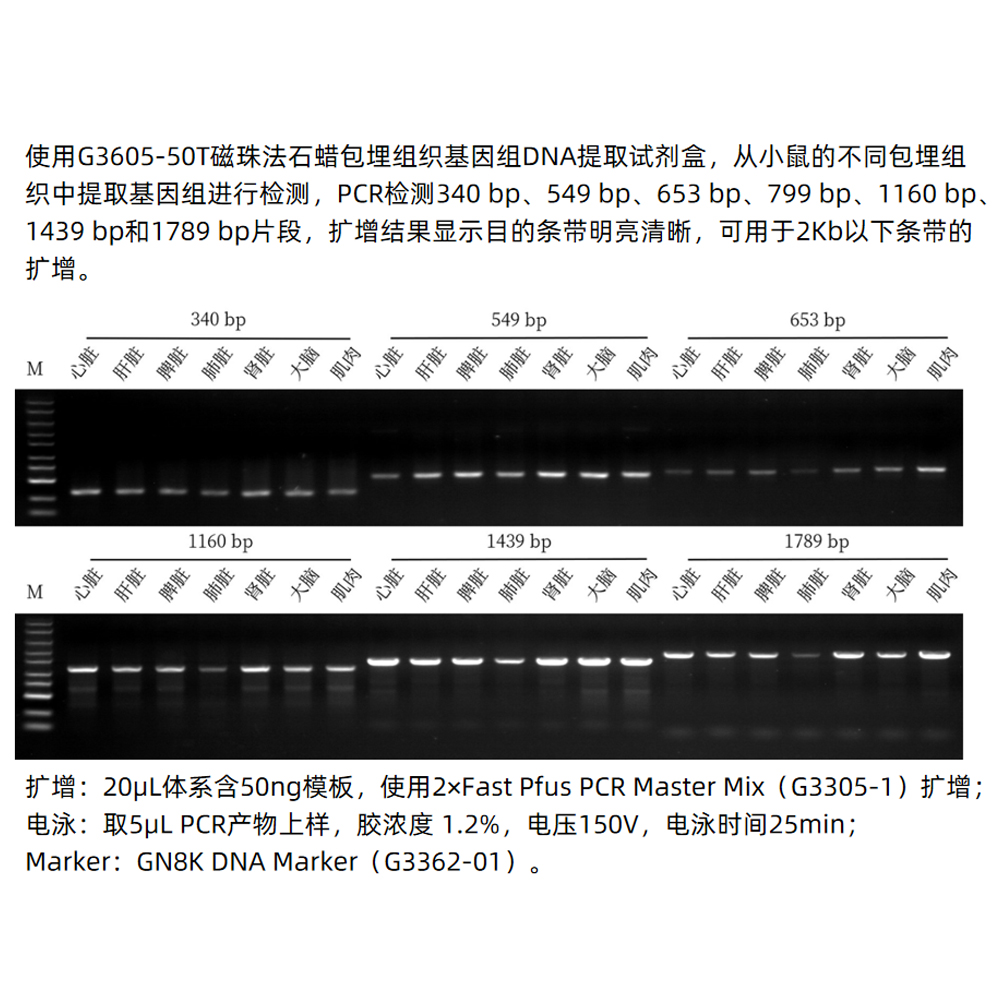
This kit selectively adsorbs plasmid DNA using a unique combination of embedded magnetic beads and alkaline lysis. It can extract up to 20 μg of high-purity plasmid DNA from 1–5 mL of overnight bacterial culture. This DNA is suitable for a wide range of molecular biology experiments such as PCR amplification, sequencing, in vitro transcription, restriction enzyme reactions, and transformation.
Storage and Transportation
RNase A is shipped on wet ice and should be stored at -20°C. All other reagents are shipped and stored at room temperature. The kit has a shelf life of 12 months.
| Component Number | Component | G3600-100T |
|---|---|---|
| G3600-1 | Solution I | 20 mL |
| G3600-2 | Solution II | 20 mL |
| G3600-3 | Neutralization Buffer | 20 mL |
| G3600-4 | RNase A | 200 μL |
| G3600-5 | SweMag Beads | 3.5 mL |
| G3600-6 | Binding Buffer | 20 mL |
| G3600-7 | Buffer SPW | 24 mL |
| G3600-8 | Buffer TE | 15 mL |
| Instructions | User Manual | 1 copy |
Here are the operating steps translated into English:
Preparation Before Use:
- Prior to use, add all of the provided RNase A to Solution I and store at 4°C.
- If Solution II exhibits precipitation, heat it at 65°C until the precipitate disappears, then mix well. (Solution II should not be exposed to the air for too long.)
- Before using Binding Buffer and SPW Buffer, add the specified amount (as indicated on the bottle) of ethanol.
- Prepare a magnetic rack and 1.5 mL sterile centrifuge tubes.
Operating Steps:
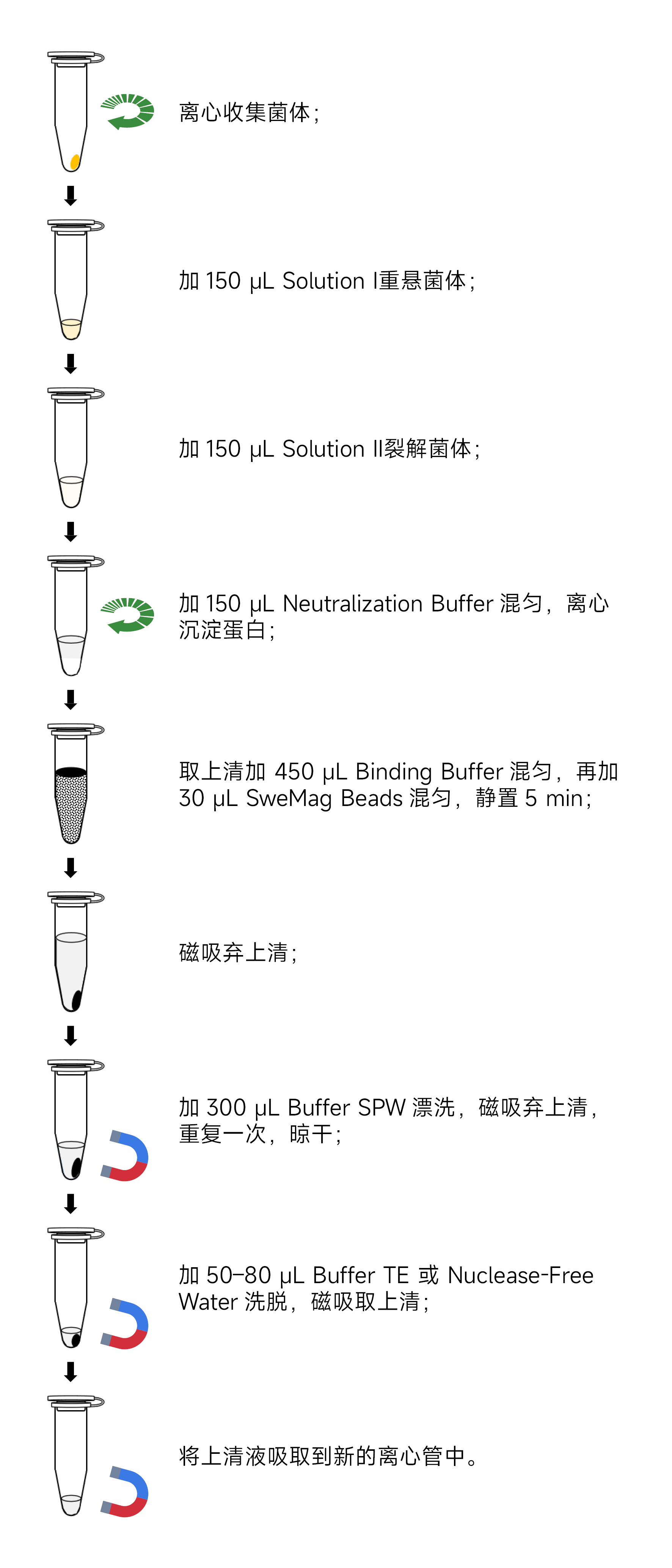
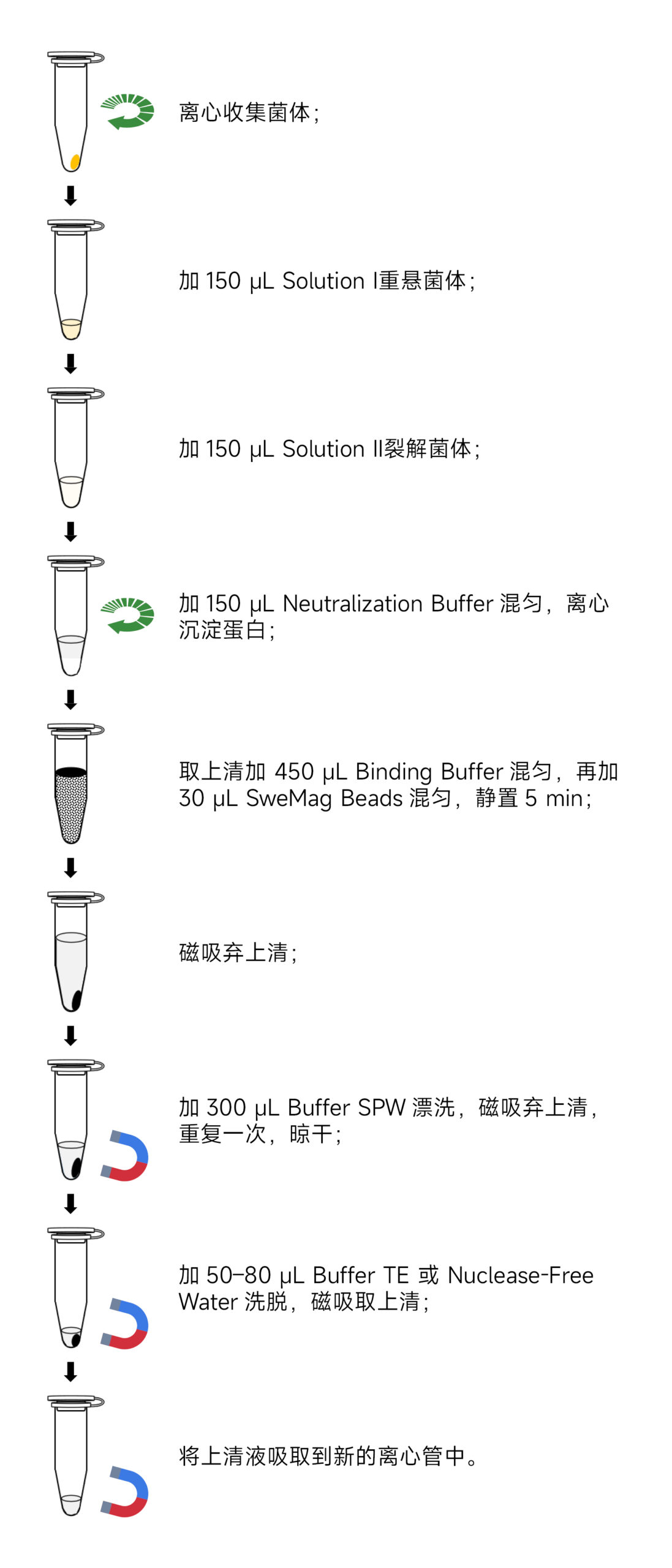
- Take 1-5 mL of overnight cultured fresh bacterial liquid, centrifuge at 12,000 rpm for 1 minute, and collect the pellet in a 1.5 mL centrifuge tube. Discard the supernatant.
- Add 150 μL of Solution I containing RNase A to the pellet. Thoroughly disperse the cells using a pipette or vortex mixer.
- Add 150 μL of Solution II. Gently invert the tube 8-10 times to thoroughly lyse the cells, forming a clear solution. (Do not exceed 5 minutes for this step.)
- Add 150 μL of pre-cooled Neutralization Buffer at 4°C. Immediately, gently invert the tube 8-10 times to mix thoroughly. (A white precipitate will form.) Centrifuge at 12,000 rpm for 10 minutes.
- Carefully transfer the supernatant to a new 1.5 mL sterile centrifuge tube. Add 450 μL of Binding Buffer. Gently invert the tube to mix, then add 30 μL of SweMag Beads (vortex SweMag Beads until evenly dispersed). Gently invert the tube a few times to ensure uniform dispersion of the magnetic beads.
- Allow the tube to stand at room temperature for 5 minutes, gently mixing with a pipette or vortex mixer 2-3 times to maintain even dispersion of the magnetic beads.
- Place the centrifuge tube on the magnetic rack and allow it to stand for 30 seconds until the magnetic beads are completely adsorbed. Gently invert the tube a few times along with the magnetic rack to wash off the magnetic beads adhering to the tube wall. Discard the supernatant (do not aspirate the magnetic beads).
- Add 300 μL of Buffer SPW. Remove the tube from the magnetic rack and gently pipette to ensure even dispersion of the magnetic beads. Place the tube back on the magnetic rack for 30 seconds, then gently invert the tube a few times along with the magnetic rack to wash off the magnetic beads and salt. Discard the supernatant (be sure to aspirate all remaining liquid in the centrifuge tube).
- Repeat step 8.
- Remove the tube cap and allow it to stand at room temperature for 5-10 minutes or at 65°C for 3-5 minutes to allow ethanol to evaporate completely (avoid overdrying the magnetic beads to prevent a decrease in nucleic acid yield).
- Remove from the magnetic rack, add 50-80 μL of Buffer TE or Nuclease-free Water, and gently pipette to ensure even dispersion of the magnetic beads. Allow to stand at room temperature for 3 minutes.
- Place the tube on the magnetic rack until the magnetic beads are completely adsorbed. Aspirate the supernatant into a new sterile centrifuge tube to obtain high-purity plasmid DNA.