Description
Product Overview
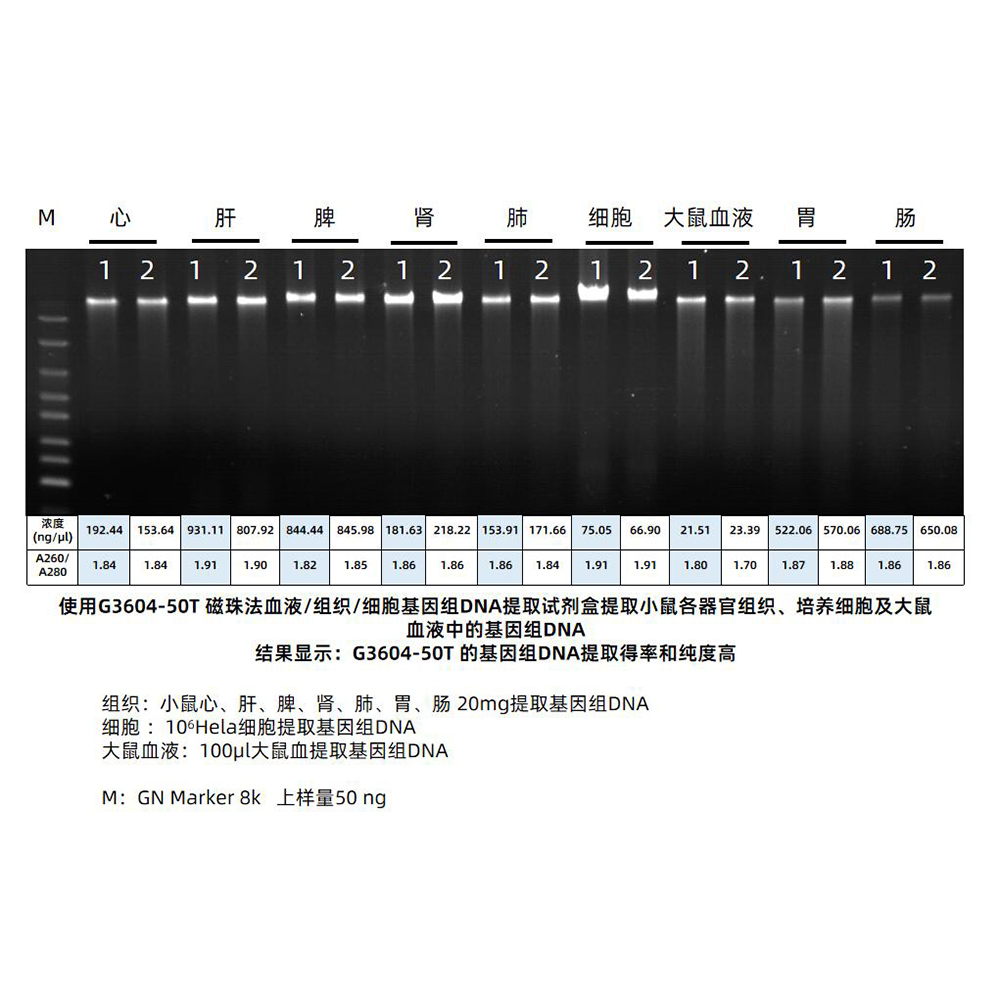
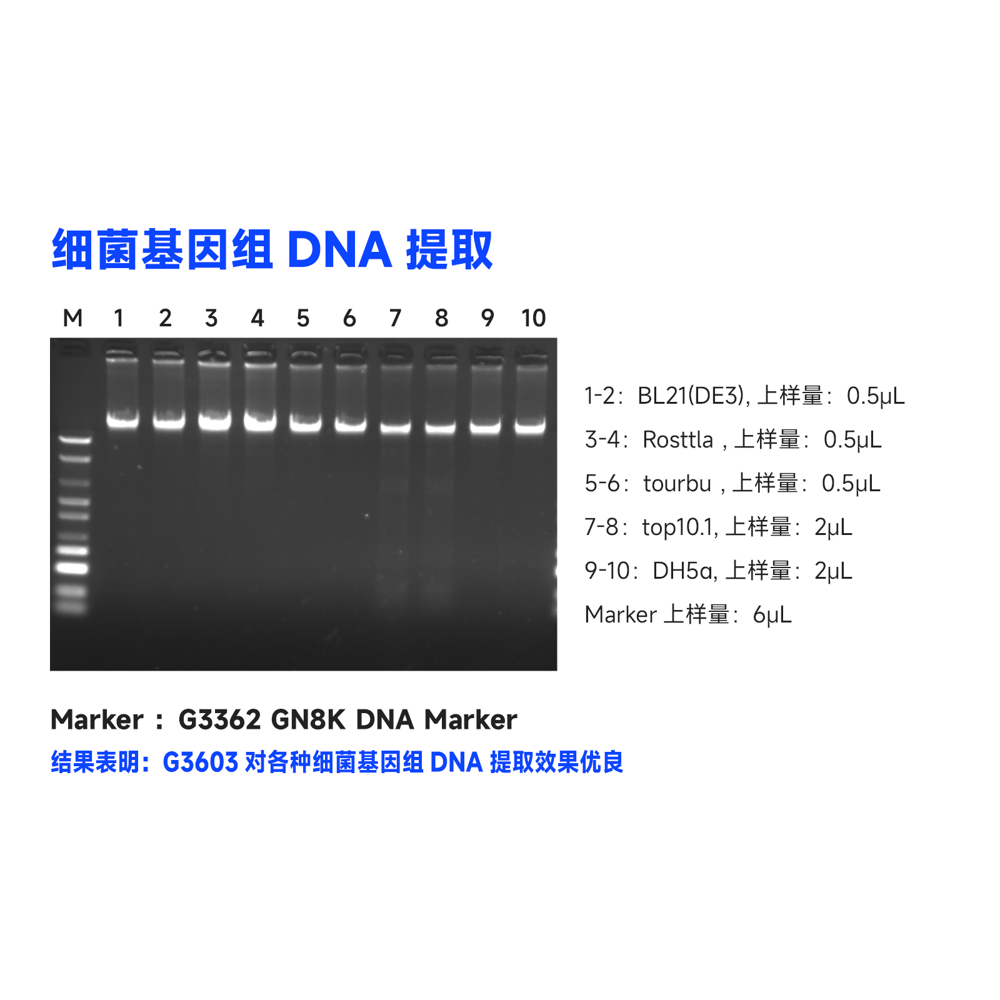
This kit utilizes specially optimized superparamagnetic beads in conjunction with specific lysis and binding solutions to selectively adsorb genomic DNA from blood, tissue, or cell samples. Impurities on the surface of the beads are removed with a washing solution, and the genomic DNA bound to the beads is eluted using an elution solution, resulting in high-quality genomic DNA. This product is suitable for the rapid purification of high-purity, high-integrity genomic DNA from various sample types, including blood, tissue, or cells. The obtained genomic DNA can be directly used in a wide range of molecular biology experiments, including PCR amplification, qPCR, enzyme digestion reactions, Southern blotting.
Product Information
- Product Name: Magnetic Bead-based Tissue/Cell/Blood Genomic DNA Extraction Kit
- Product Number: G3604-50T
- Specifications: 50T
Product Overview
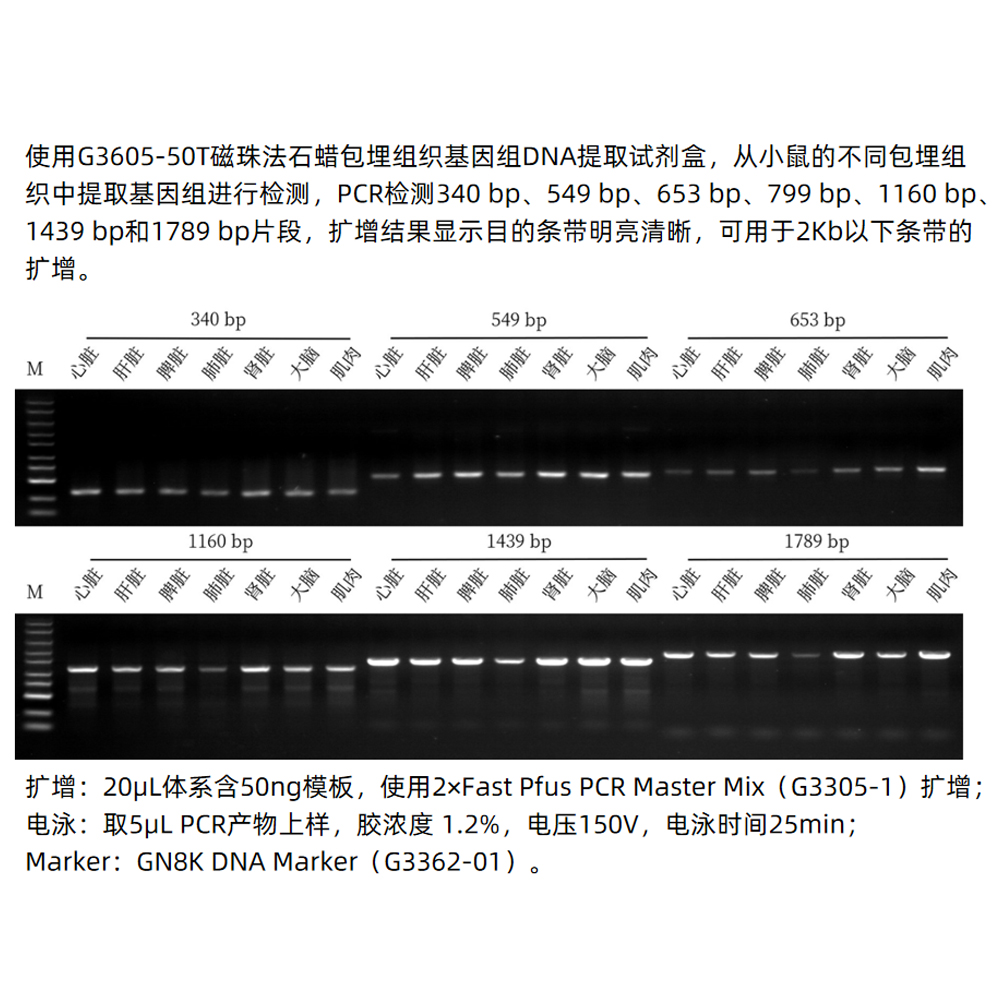
This kit utilizes a specially optimized lysis buffer to release genomic DNA from tissue/cells/blood samples. The genomic DNA is selectively adsorbed from the lysate using superparamagnetic beads in the presence of a binding solution. After removing impurities with a washing solution, the genomic DNA is eluted using an elution solution, resulting in high-quality genomic DNA. This product is suitable for the rapid extraction of high-purity, intact genomic DNA from various sources, including animal tissues, cultured cells, and whole blood samples (with anticoagulants). It can efficiently extract genomic DNA from 2-30 mg of animal tissue, 10^6-10^7 freshly cultured cell suspensions, or 5-100 μL of nucleated or non-nucleated whole blood (with anticoagulants). The obtained genomic DNA can be directly used in downstream molecular biology experiments such as PCR, enzyme digestion, and hybridization.
Storage and Transportation
- Proteinase K and RNase A are shipped on wet ice and should be stored at -20°C. Other reagents are shipped and stored at room temperature. The kit has a shelf life of 12 months.
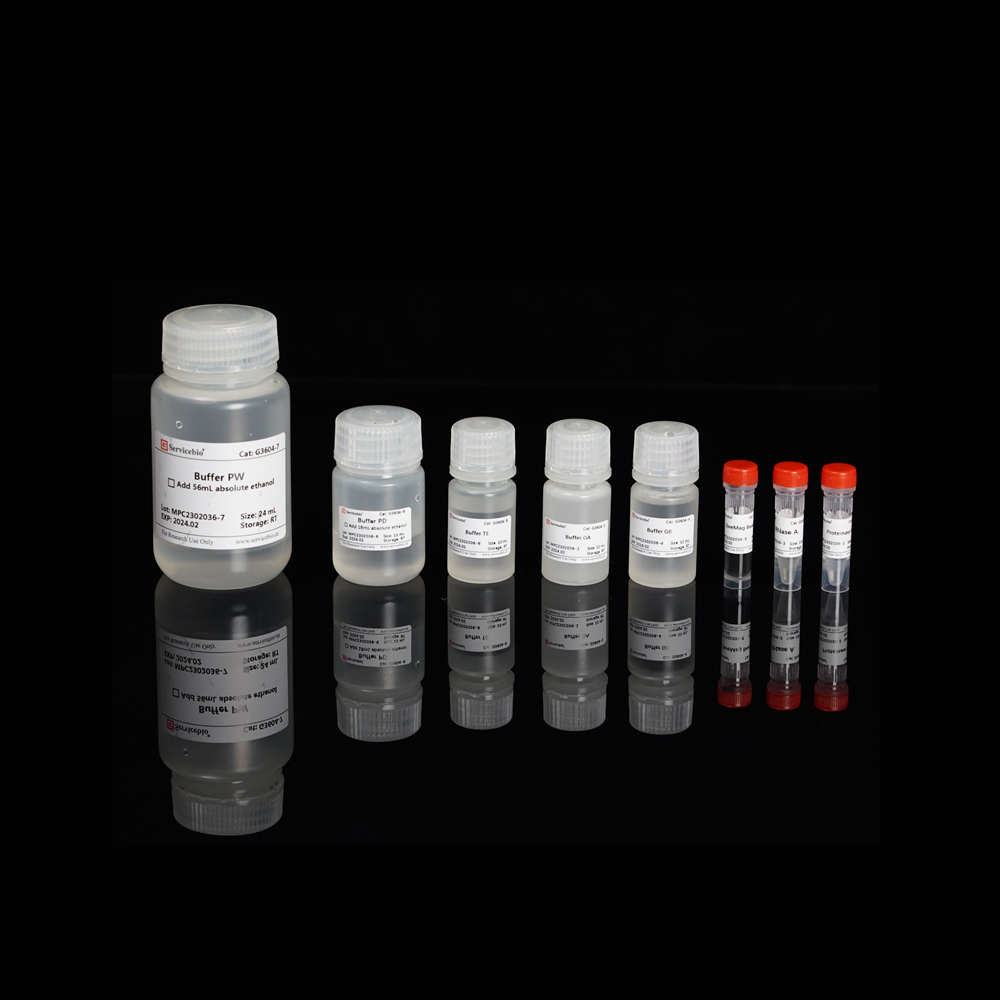
Here is the component list for the Magnetic Bead-based Tissue/Cell/Blood Genomic DNA Extraction Kit (Product Number G3604-50T):
| Component Number | Component | G3604-50T |
|---|---|---|
| G3604-1 | Buffer GA | 12 mL |
| G3604-2 | Proteinase K | 1 mL |
| G3604-3 | RNase A | 200 µL |
| G3604-4 | Buffer GB | 12 mL |
| G3604-5 | SweMag Beads | 1 mL |
| G3604-6 | Buffer PD | 12 mL (add 18 mL ethanol before use) |
| G3604-7 | Buffer PW | 24 mL (add 56 mL ethanol before use) |
| G3604-8 | Buffer TE | 10 mL |
| Instruction Manual | 1 copy |
Please note that the specified volumes of ethanol should be added to Buffer PD and Buffer PW before use.
Here are the operational steps for the Magnetic Bead-based Tissue/Cell/Blood Genomic DNA Extraction Kit:
Preparation before use:
- Prepare a magnetic rack and anhydrous ethanol.
- If Buffer GA has precipitated, heat it to 65°C to dissolve it, then allow it to return to room temperature before use.
- Before use, add 18 mL of anhydrous ethanol to Buffer PD and 56 mL of anhydrous ethanol to Buffer PW.
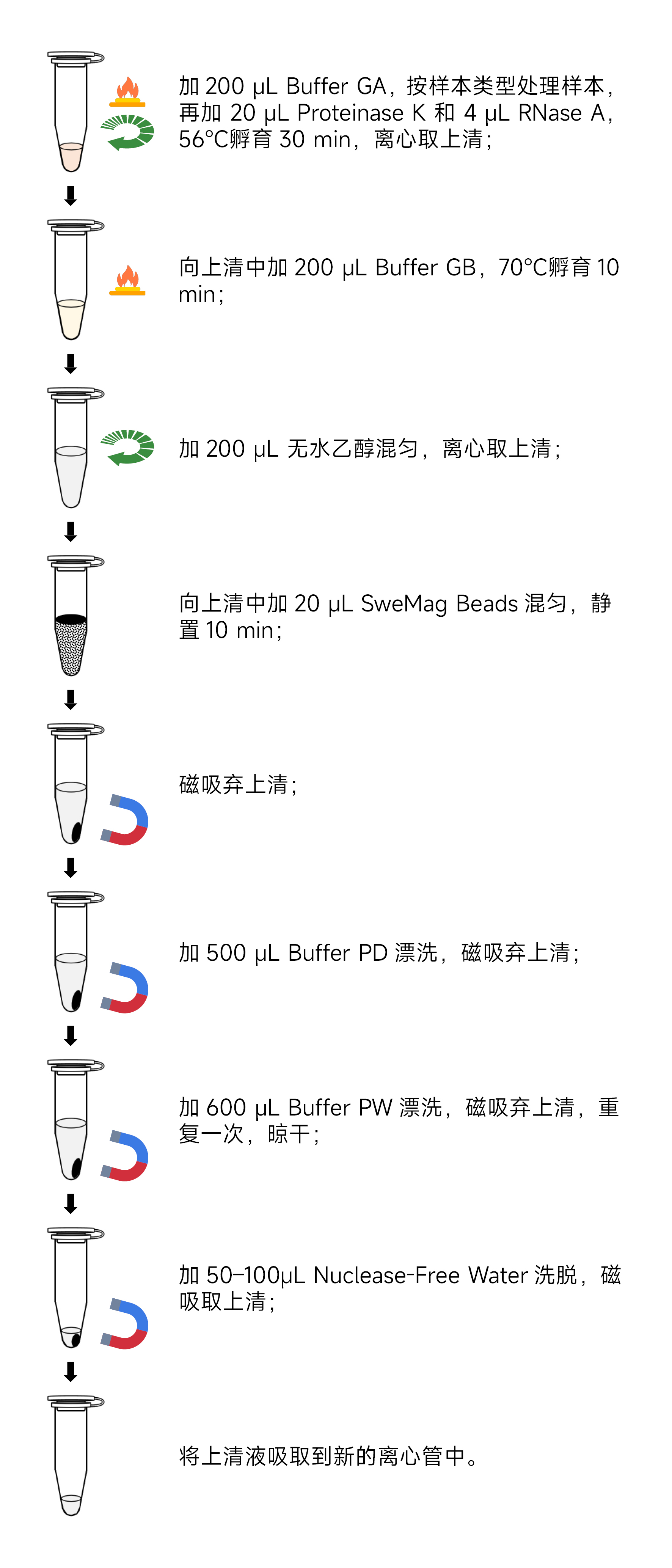
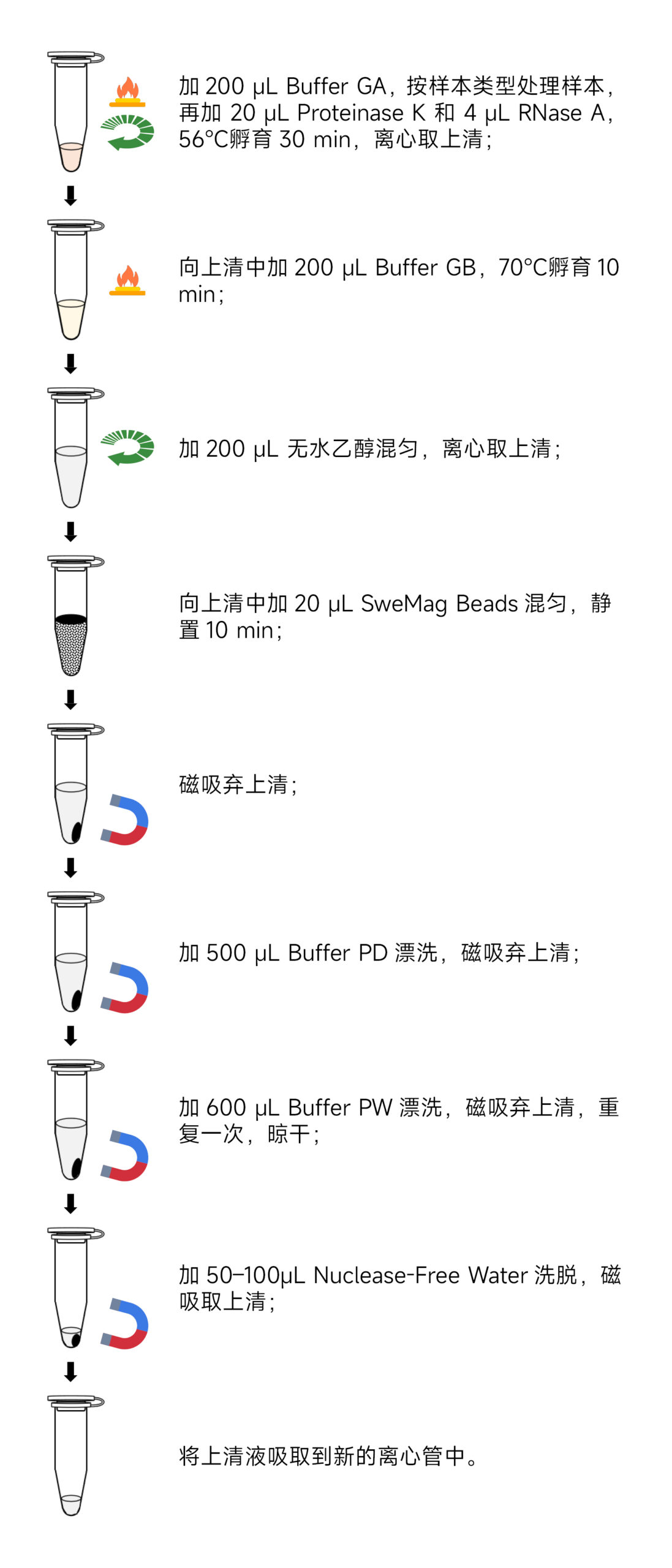
- Certainly! Here are the experimental steps translated into English:Experimental Steps:
- Sample Preparation: a. Take 2-30 mg of fresh or ultra-low-temperature frozen animal tissue, place it in a 1.5 mL centrifuge tube or a specialized grinding tube containing 2-3 3 mm grinding beads (recommended G0203-150G). Add 200 μL of Buffer GA. Use a tissue grinder (recommended KZ-5F-3D) to thoroughly grind the tissue into a homogenous paste at room temperature. Ensure that the tissue is completely homogenized, as incomplete homogenization will affect DNA yield and quality. b. Take 106-107 cell suspensions, place them in a 1.5 mL centrifuge tube, centrifuge at 1,000 rpm for 5 minutes, and gently aspirate the supernatant. Add 200 μL of Buffer GA and vortex to mix. c. Take 50-100 μL of nucleated whole blood (with anticoagulants) in a 1.5 mL centrifuge tube, and supplement it with Buffer GA to a total volume of 200 μL. Vortex to mix (if processing a larger volume of blood, add 3 times the volume of red blood cell lysis buffer (recommended G2015) to the sample, invert mix, leave at room temperature for 5 minutes, invert mix 2-3 times during this period, and centrifuge at 3000 rpm for 10 minutes. Gently aspirate the supernatant, avoiding white cell precipitate). d. Take 5-20 μL of nucleated whole blood (with anticoagulants) and place it in a 1.5 mL centrifuge tube. Supplement it with Buffer GA to a total volume of 200 μL. Mix gently using a pipette.
- Add 20 μL of Proteinase K and 4 μL of RNase A, and incubate the samples at 56°C for 30 minutes. Mix gently every 10 minutes to accelerate tissue lysis. For materials that are difficult to lyse, you can extend the lysis time as needed.
- Centrifuge at 12,000 rpm for 2 minutes and transfer the supernatant to a Nuclease-free centrifuge tube, avoiding aspirating the precipitate (skip this step when extracting genomic DNA from cell/blood samples).
- Add 200 μL of Buffer GB to the centrifuge tube, invert to mix, and incubate at 70°C for 10 minutes.
- Add 200 μL of anhydrous ethanol to the centrifuge tube, invert to mix. At this point, there may be a flocculent precipitate. Centrifuge at 12,000 rpm for 2 minutes and transfer the clear supernatant to a Nuclease-free centrifuge tube, avoiding aspirating the precipitate.
- Add 20 μL of SweMag Beads to the centrifuge tube (make sure to vortex SweMag Beads thoroughly before use), and use a pipette to gently mix until the beads are evenly dispersed.
- Allow it to stand at room temperature for 10 minutes, during which mix gently 2-3 times using a pipette to keep the beads suspended.
- Place the centrifuge tube on a magnetic rack and allow it to stand for 30 seconds until the supernatant becomes clear. Discard the supernatant.
- Add 500 μL of Buffer PD to the tube, remove it from the magnetic rack, and use a pipette to mix until the beads are evenly dispersed. Place the tube on the magnetic rack for 30 seconds until the supernatant becomes clear. Discard the supernatant.
- Add 600 μL of Buffer PW to the tube, remove it from the magnetic rack, and use a pipette to mix until the beads are evenly dispersed. Place the tube on the magnetic rack for 30 seconds until the supernatant becomes clear. Discard the supernatant.
- Repeat step 10.
- Leave the tube open at room temperature for 5-10 minutes to allow ethanol to completely evaporate (avoid excessive drying of the beads, as it may affect nucleic acid yield).
- Remove it from the magnetic rack, add 50-100 μL of Buffer TE or Nuclease-free Water, and use a pipette to gently mix until the beads are evenly dispersed. Allow it to stand at room temperature for 5 minutes.
- Place the tube on the magnetic rack until the beads are completely adsorbed, and then aspirate the clear supernatant into a new Nuclease-free 1.5 mL centrifuge tube to obtain high-purity DNA.Precautions:
- Before proceeding, please read this product manual carefully.
- Fresh laboratory materials should be used whenever possible to ensure that the extracted genomic DNA is not degraded.
- Samples should be avoided from repeated freeze-thaw cycles, as this may lead to a decrease in DNA yield.
- Tissue materials should not exceed the maximum starting amount, and they should be thoroughly lysed to avoid potential impacts on yield.
- Avoid freezing the magnetic bead suspension during storage. Magnetic beads tend to settle, so ensure thorough mixing by shaking or vortexing before use to maintain a uniform suspension.
- Mix the reagents in the sample thoroughly before adding magnetic beads.
- Avoid prolonged drying of the magnetic beads, as it may affect DNA elution efficiency.