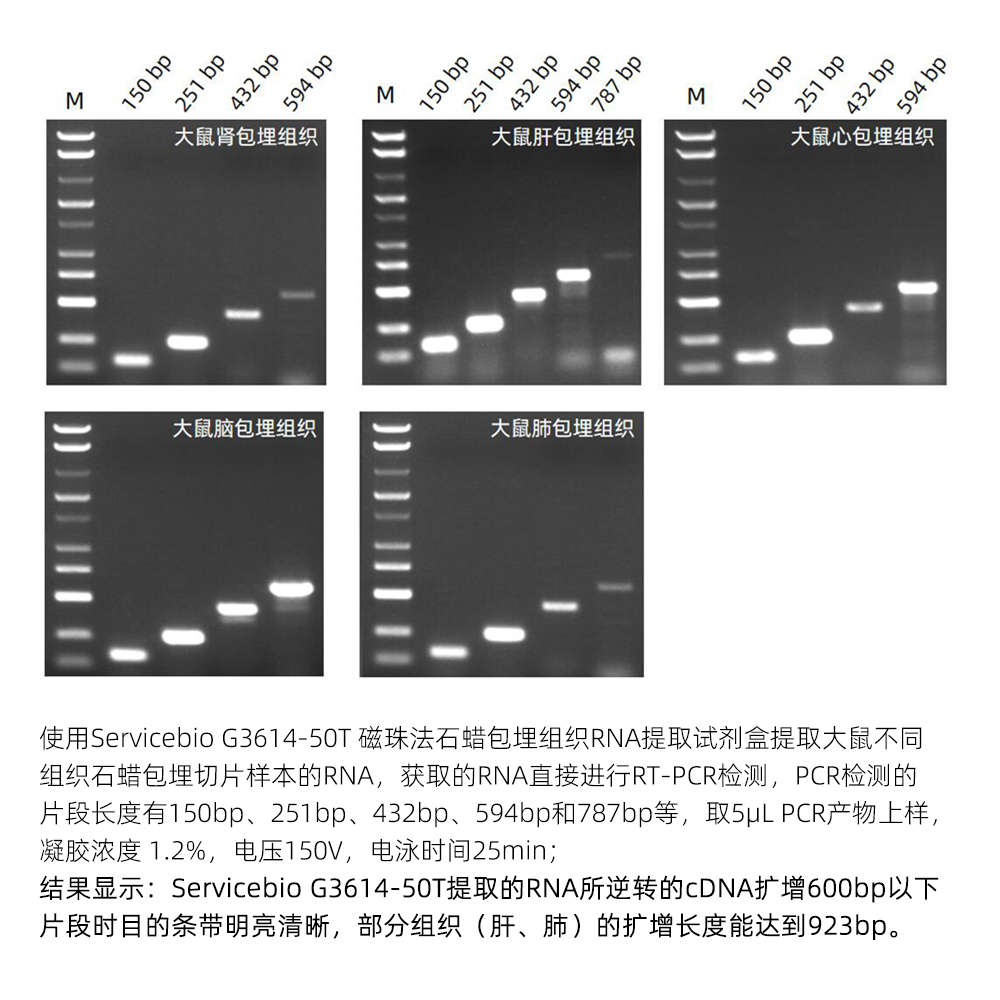
Description
Product Information
Product Name: Magnetic Bead-based Paraffin-embedded Tissue Genomic DNA Extraction Kit
Product Number: G3605-50T
Specifications: 50 tests
Product Description:
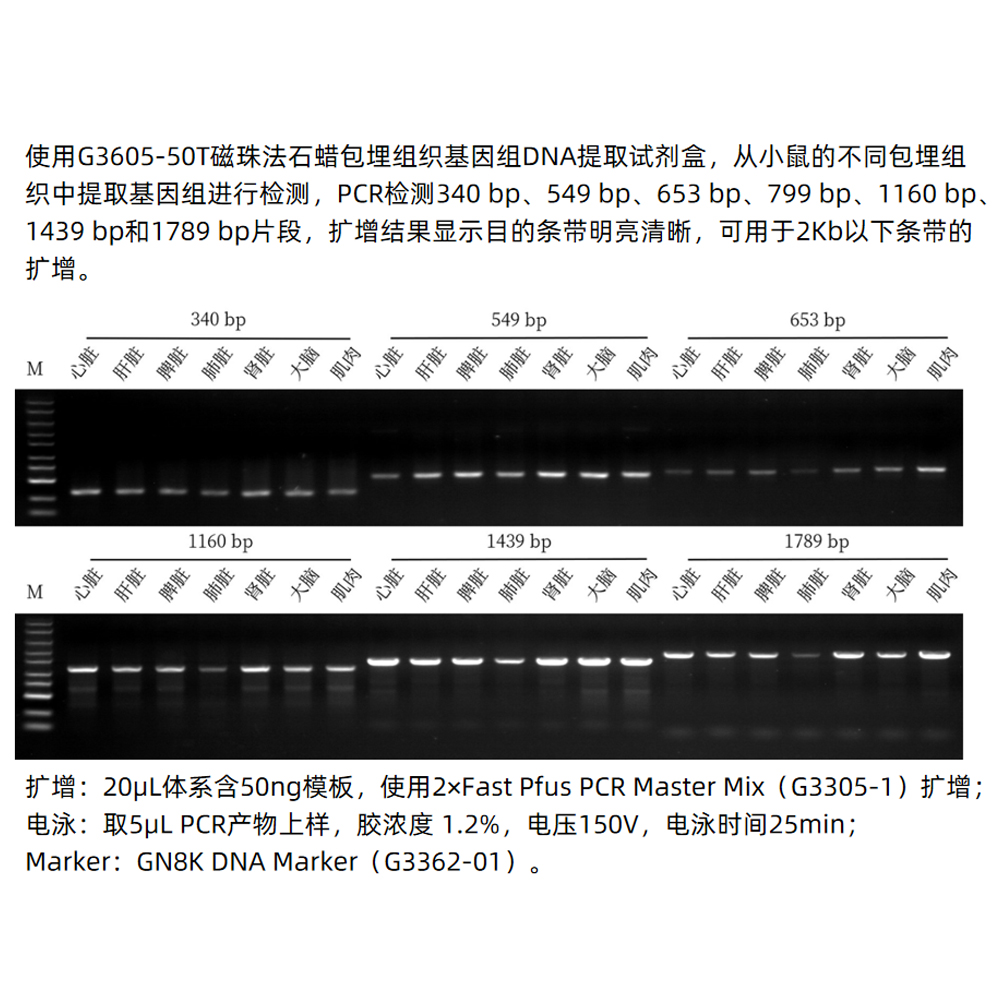
This kit utilizes a unique dewaxing reagent that efficiently removes paraffin blocks surrounding paraffin-embedded tissues, eliminating the need for toxic reagents like xylene. Through specially optimized lysis buffers, it effectively releases genomic DNA from tissues. It also takes advantage of the superparamagnetic bead’s specific binding to genomic DNA, allowing for the rapid and efficient extraction of genomic DNA from tissues. The obtained genomic DNA has high yield and purity, making it suitable for various molecular biology experiments such as PCR, Real-Time PCR, SNP genotyping, and more.
Storage and Transportation:
RNase A and Proteinase K are shipped on wet ice and should be stored at -20°C. All other reagents can be shipped and stored at room temperature. The kit has a shelf life of 12 months.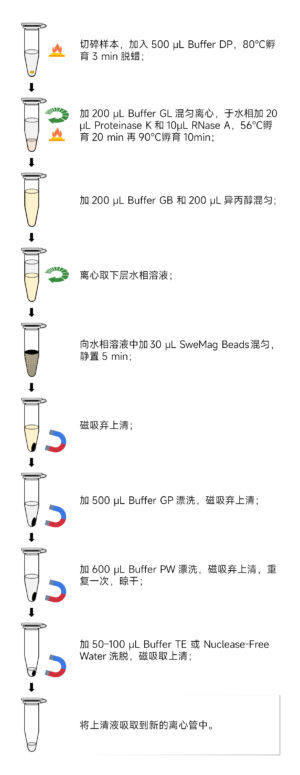
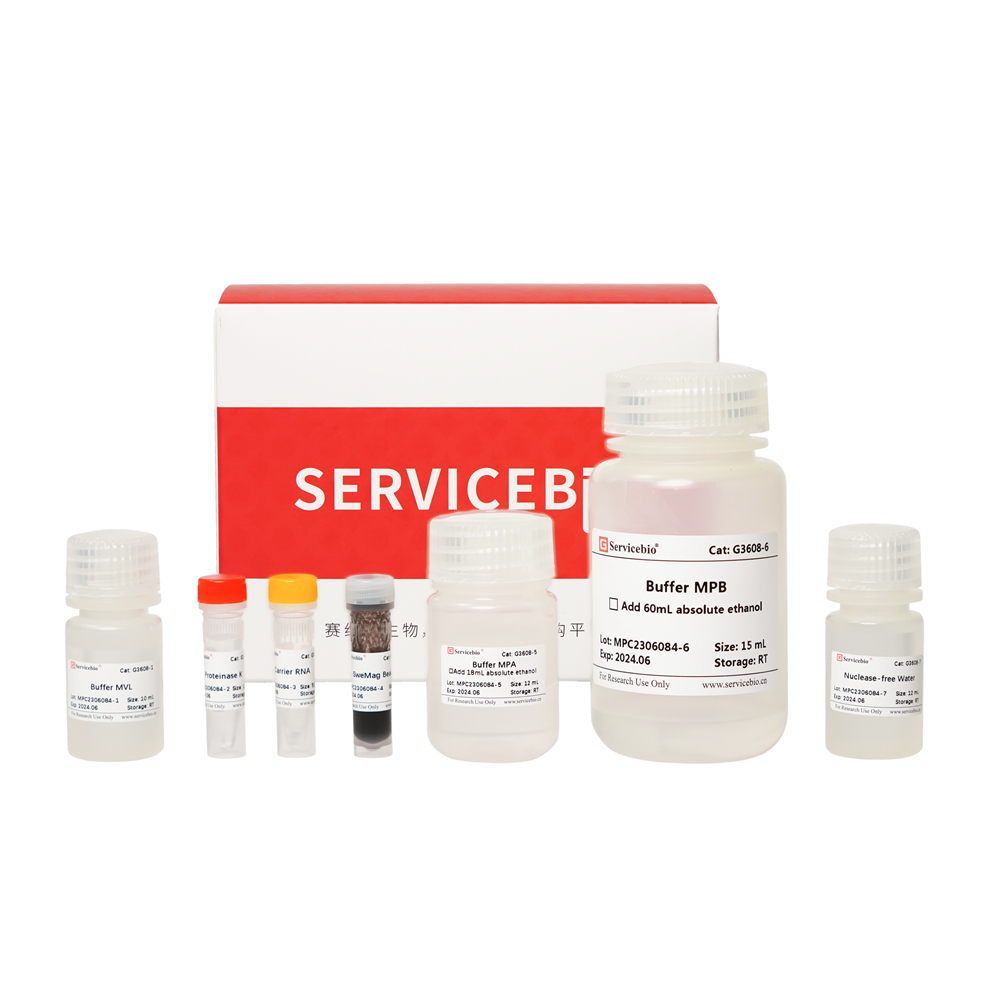
Here’s the component list for the Magnetic Bead-based Paraffin-embedded Tissue Genomic DNA Extraction Kit (Product Number: G3605-50T):
- G3605-1: Buffer DP (30 mL)
- G3605-2: Buffer GL (10 mL)
- G3605-3: Proteinase K (1 mL)
- G3605-4: RNase A (500 μL)
- G3605-5: Buffer GB (10 mL)
- G3605-6: SweMag Beads (1.5 mL)
- G3605-7: Buffer GP (9 mL, add 21 mL of ethanol before use)
- G3605-8: Buffer PW (24 mL, add 56 mL of ethanol before use)
- G3605-9: Buffer TE (10 mL)
You will also receive 1 copy of the instruction manual with the kit
Here are the steps for using the Magnetic Bead-based Paraffin-embedded Tissue Genomic DNA Extraction Kit (Product Number: G3605-50T):
Before you begin, prepare the following:
- Preheat water baths or metal blocks to 80°C, 56°C, and 90°C.
- If Buffer GL and Buffer GB have precipitated, heat them to 65°C to dissolve. Use them at room temperature after they have returned to a liquid state.
- Add 21 mL of ethanol to Buffer GP and 56 mL of ethanol to Buffer PW. Mix well before use.
- Have a magnetic stand and isopropanol ready.
Procedure:
Sample Preparation:
- For paraffin-embedded tissue sections: Take 5-8 paraffin-embedded tissue sections (1×1 cm²) and collect tissue fragments using a sterile surgical blade. Place them in a 1.5 mL centrifuge tube.
- For paraffin-embedded tissue blocks: Scrape off approximately 30 mg of tissue sample with a sterile surgical blade, ensuring the removal of excess paraffin. Cut the sample into smaller pieces and place them in a 1.5 mL centrifuge tube.
- For formalin-fixed tissues: Dehydrate the tissue surface with filter paper, take approximately 30 mg of the sample, cut it into pieces, and place it in a 1.5 mL centrifuge tube. Add 500 μL of PBS buffer (pH 7.4). Vortex for 10 seconds and centrifuge at 12,000 rpm at room temperature for 1 minute. Discard the supernatant. Repeat this step 3 times.
DNA Extraction Steps: 4. Add 500 μL of Buffer DP to the centrifuge tube containing the sample. Incubate at 80°C for 3 minutes and vortex for 10 seconds while hot.
- Add 200 μL of Buffer GL to the tube. Vortex to mix, then centrifuge at 12,000 rpm at room temperature for 1 minute. The solution will form two layers (an upper oil phase and a lower aqueous phase).
- Add 20 μL of Proteinase K and 10 μL of RNase A to the lower aqueous phase. Gently pipette to mix. Incubate at 56°C for 20 minutes (you can extend the incubation time to 20 minutes if there is significant undigested tissue).
- Transfer the tube to 90°C and incubate for 10 minutes. Cool to room temperature.
- Add 200 μL of Buffer GB and 200 μL of isopropanol to the sample. Vortex to mix.
- Centrifuge at 12,000 rpm at room temperature for 1 minute. The solution will form two layers.
- Transfer the lower aqueous phase to a new 1.5 mL centrifuge tube. Add 30 μL of SweMag Beads (vortex them to disperse evenly) and gently pipette to ensure even dispersion.
- Allow the tube to stand at room temperature for 5 minutes, gently pipetting 2-3 times during this period to keep the beads evenly dispersed.
- Place the tube on a magnetic stand for 30 seconds to allow the beads to adsorb to the tube wall. Once the supernatant is clear, remove and discard it, being careful not to aspirate the beads.
- Add 500 μL of Buffer GP to the tube. Use a pipette to gently pipette and ensure even dispersion of the beads. Place the tube on a magnetic stand for 30 seconds to allow the beads to adsorb to the tube wall. Once the supernatant is clear, remove and discard it, being careful not to aspirate the beads.
- Remove the tube from the magnetic stand and add 600 μL of Buffer PW. Use a pipette to gently pipette and ensure even dispersion of the beads. Place the tube on a magnetic stand for 30 seconds to allow the beads to adsorb to the tube wall. Once the supernatant is clear, remove and discard it, being careful not to aspirate the beads.
- Repeat step 14.
- Leave the tube open at room temperature for 5-10 minutes or at 65°C for 3-5 minutes to allow ethanol to completely evaporate (do not overdry the beads as it may affect DNA yield).
- Remove the tube from the magnetic stand. Add 50-100 μL of Buffer TE or nuclease-free water. Gently pipette to ensure even dispersion of the beads. Allow to stand at room temperature for 3-5 minutes, gently pipetting 2-3 times during this period.
- Place the tube on the magnetic stand until the beads are completely adsorbed. Transfer the supernatant to a new centrifuge tube to obtain high-purity genomic DNA.
Please note that these instructions assume you are using the Magnetic Bead-based Paraffin-embedded Tissue Genomic DNA Extraction Kit (Product Number: G3605-50T). Follow the kit’s specific instructions and safety guidelines provided in the manual.
Here are the safety precautions and guidelines for using the Magnetic Bead-based Paraffin-embedded Tissue Genomic DNA Extraction Kit:
Safety Precautions:
- Before starting, carefully read the product instruction manual.
- The yield and integrity of DNA extracted using this kit depend on sample type, fixation time, fixation conditions, and sample storage time. It is recommended to fix samples for a duration of 8-24 hours. If fixation time exceeds 24 hours or if samples are stored for more than 1 year, excessive DNA fragmentation may occur, making it difficult to amplify the target bands.
- Ensure that samples are completely dehydrated before embedding to prevent residual formalin from affecting subsequent experiments.
- When sampling paraffin-embedded tissues, try to remove excess paraffin and cut the samples into small pieces. Do not exceed 30 mg of sample weight, as excessive sample size can result in incomplete lysis, affecting DNA yield.
- Magnetic beads may settle, so it’s important to shake or vortex them well before use. Avoid freezing the magnetic bead suspension during storage.
- Before eluting genomic DNA, ensure that ethanol has completely evaporated to avoid any residual ethanol that could impact downstream experiments. However, avoid overdrying the magnetic beads for fear of affecting genomic DNA elution efficiency.
- When using the extracted genomic DNA as a template for PCR amplification, it is recommended to perform template concentration gradient tests to select the optimal template concentration for amplification.
- For your safety and health, wear a lab coat and disposable gloves while performing the procedure.
Note: This product is intended for research purposes only and is not intended for clinical diagnosis.