Description
Product Information
- Product Name: Magnetic Bead-Based Bacterial Total RNA Extraction Kit
- Product Number: G3617-50T
- Specifications: 50T
Product Description
This kit is designed for RNA extraction from both Gram-positive and Gram-negative bacteria. It utilizes the reversible binding properties of superparamagnetic beads combined with a carefully optimized lysis buffer system to rapidly and effectively isolate RNA from bacteria. The extraction process can be completed within 30-40 minutes, resulting in high RNA integrity, purity, and yield. This kit is suitable for various downstream molecular biology experiments, including RT-PCR, Real-Time PCR, chip analysis, Northern Blot, Dot Blot, PolyA selection, in vitro translation, RNase protection analysis, and molecular cloning.
Storage and Transportation
- Lysozyme (50 mg/mL), DTT solution, and DNase are transported on wet ice and should be stored at -20℃.
- Other reagents are transported at room temperature and should be stored at room temperature.
- The kit is valid for 12 months.
Please note that the provided information is for research purposes only and is not intended for clinical diagnosis.
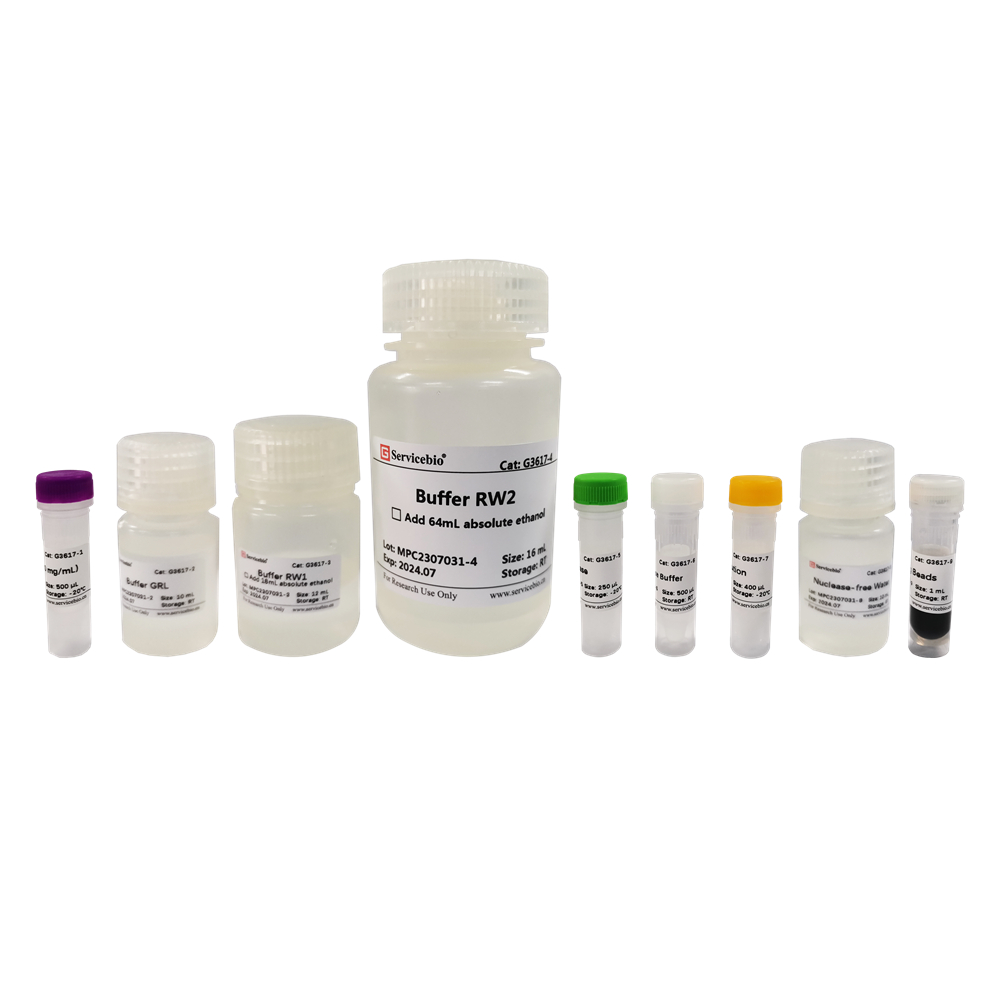
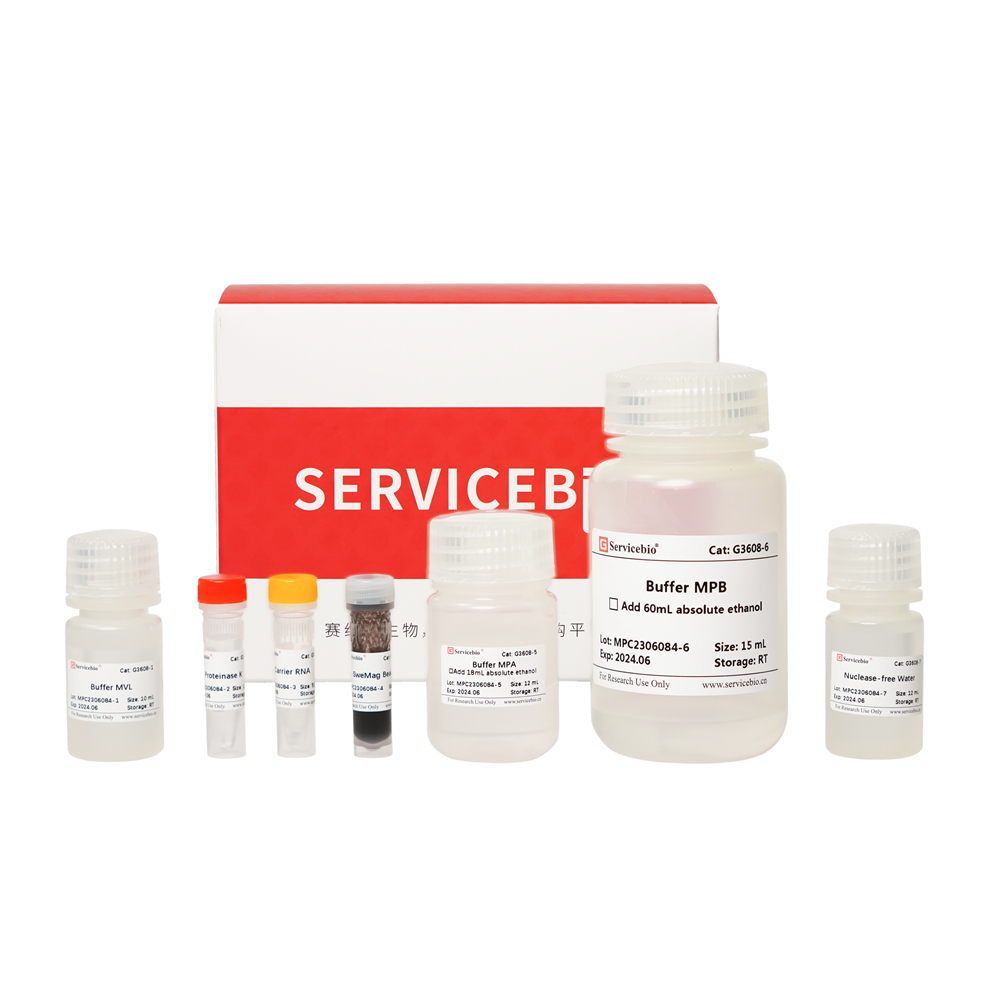
| Component Number | Component | G3617-50T |
| G3617-1 | Lysozyme(50 mg/mL) | 500 μL |
| G3617-2 | Buffer GRL | 10 mL |
| G3617-3 | Buffer RW1 | 12 mL |
| G3617-4 | Buffer RW2 | 16 mL |
| G3617-5 | DNase | 250 μL |
| G3617-6 | 10×DNase Buffer | 500 μL |
| G3617-7 | DTT Solution | 400 μL |
| G3617-8 | Nuclease-free Water | 10 mL |
| G3617-9 | SweMag Beads | 1 mL |
| 说明书 | 1份 | |
Before You Begin
- Please prepare 10 mM Tris-HCl (pH 8.0) for resuspending bacterial pellets and diluting lysozyme.
- If extracting RNA from Gram-positive bacteria, preheat a water bath or heat block to 37°C.
- If Buffer GRL has precipitated, heat and dissolve it at 37°C. Use it once it has returned to room temperature.
- Before use, add DTT Solution to Buffer GRL to a final concentration of 4%. This means adding 40 μL of DTT Solution to every 1 mL of Buffer GRL. It is best to prepare this mixture fresh. Buffer GRL with added DTT Solution can be stored at 4°C for up to one week.
- Prepare DNase working solution fresh before use.
- Before use, add 18 mL of ethanol to Buffer RW1, mix well, and then use.
- Before use, add 64 mL of ethanol to Buffer RW2, mix well, and then use.
Operating Steps
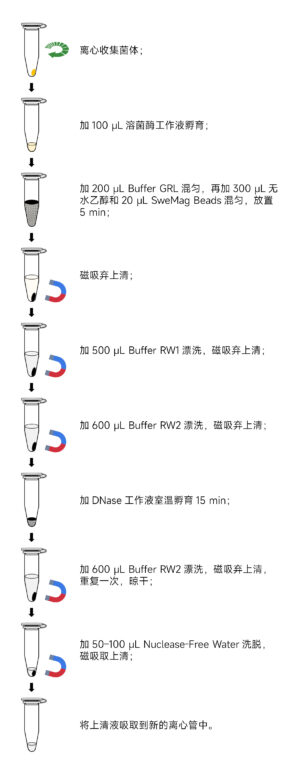
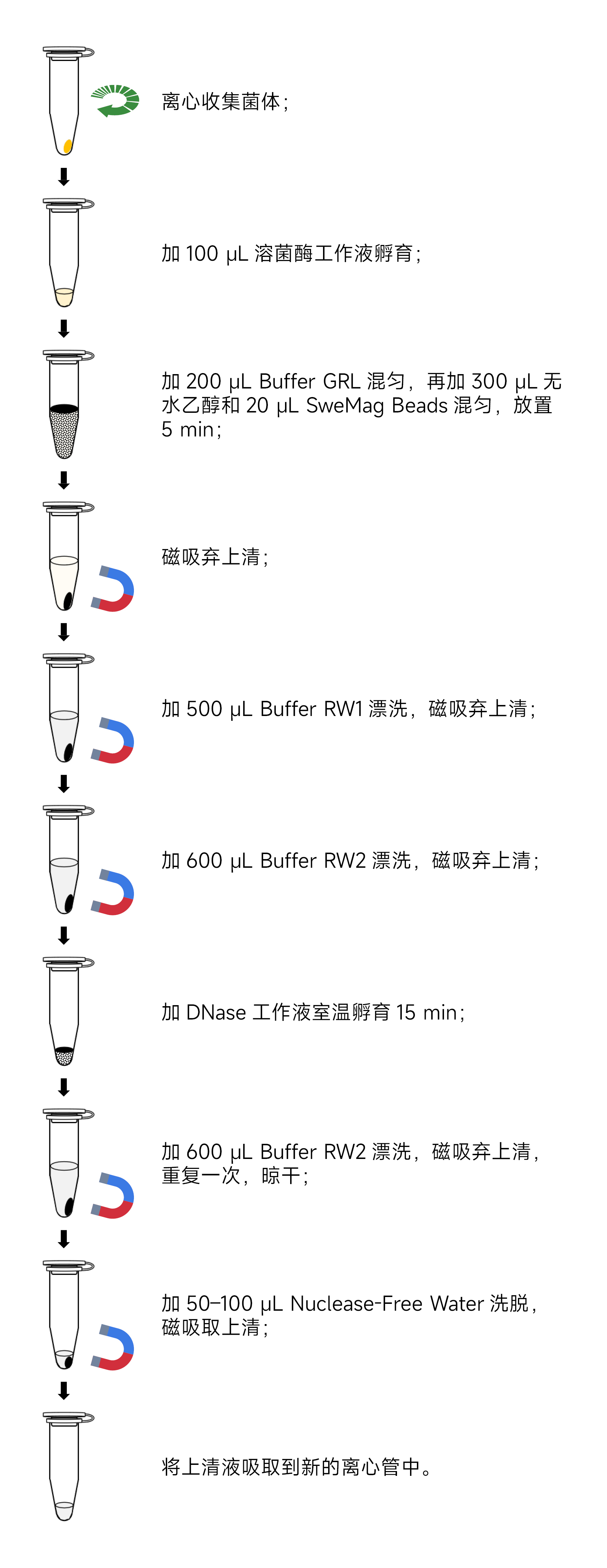
- Sample PreparationI. For Gram-negative bacteria, take 1-3 mL of bacterial culture (total bacteria not exceeding 1×10^9 cells) and centrifuge at 12,000 rpm (~13,400×g) for 2 minutes to collect bacterial cells.Note: Ensure complete removal of the supernatant to prevent interference with bacterial cell wall digestion.
Note: Do not use an excessive amount of bacterial cells. For an OD600=1 culture, it is recommended to use 1 mL of bacterial culture.
- Resuspend the Bacterial PelletResuspend the bacterial pellet in 100 μL of 10 mM Tris-HCl (pH 8.0) containing lysozyme, with the lysozyme concentration and incubation conditions as indicated in the table below:
Bacterial Type Lysozyme Concentration Incubation Temperature Incubation Time Gram-Negative (G-) Bacteria 0.4 mg/mL Room Temperature 3-5 minutes Gram-Positive (G+) Bacteria 5 mg/mL 37°C 5-10 minutes - Add 200 μL of Buffer GRL (ensure DTT Solution has been added before use) to the bacterial suspension and vortex to mix.
- Add 300 μL of anhydrous ethanol to the tube and mix by pipetting (precipitation may occur).
- Add 20 μL of SweMag Beads (vortex SweMag Beads until well-dispersed before use) to the mixture and pipette to ensure even distribution of the magnetic beads. Allow the mixture to sit at room temperature for 5 minutes, gently pipetting 2-3 times during this period to maintain even dispersion of the beads.
- Place the tube on a magnetic stand for 30 seconds to allow the magnetic beads to adsorb to the tube wall. Once the supernatant is clear, remove and discard the supernatant (do not aspirate the magnetic beads). Take the tube off the magnetic stand.
- Add 500 μL of Buffer RW1 to the tube and pipette to ensure even distribution of the magnetic beads. Then, place the tube on the magnetic stand for 30 seconds to allow the beads to adsorb to the tube wall. Once the supernatant is clear, remove and discard the supernatant (do not aspirate the magnetic beads). Remove the tube from the magnetic stand.
- Add 600 μL of Buffer RW2 to the tube and pipette to ensure even distribution of the magnetic beads. Place the tube on the magnetic stand for 30 seconds to allow the beads to adsorb to the tube wall. Once the supernatant is clear, remove and discard the supernatant (do not aspirate the magnetic beads). Remove the tube from the magnetic stand.
- Prepare the DNase working solution by combining 85 μL of Nuclease-free Water, 10 μL of 10× DNase Buffer, and 5 μL of DNase in a new Nuclease-free centrifuge tube. Gently pipette to mix.
- Add the DNase working solution to the tube along the tube wall. Scrape the magnetic beads off the tube wall and gently pipette to mix. Leave at room temperature for 15 minutes, gently pipetting 2-3 times during this period.
- Add 600 μL of Buffer RW2 to the tube and pipette to ensure even distribution of the magnetic beads. Place the tube on the magnetic stand for 30 seconds to allow the beads to adsorb to the tube wall. Once the supernatant is clear, remove and discard the supernatant (do not aspirate the magnetic beads).
- Repeat step 11.
- Open the tube cap and place it in a laminar flow hood for 5-10 minutes to allow ethanol to completely evaporate (avoid overdrying the magnetic beads, which can affect nucleic acid yield).
- Remove the tube from the magnetic stand. Add 50-100 μL of Nuclease-free Water to the tube and gently pipette to ensure even distribution of the magnetic beads. Allow it to sit at room temperature for 3-5 minutes, gently pipetting 2-3 times during this period.
- Place the tube on the magnetic stand until the magnetic beads are completely adsorbed. Transfer the supernatant to a new centrifuge tube to obtain high-purity RNA.
- Store the obtained RNA solution at -80°C.