Description
Product Information
- Product Name: Magnetic Bead-Based Paraffin-Embedded Tissue RNA Extraction Kit
- Product Number: G3614-50T
- Specifications: 50T
Product Description
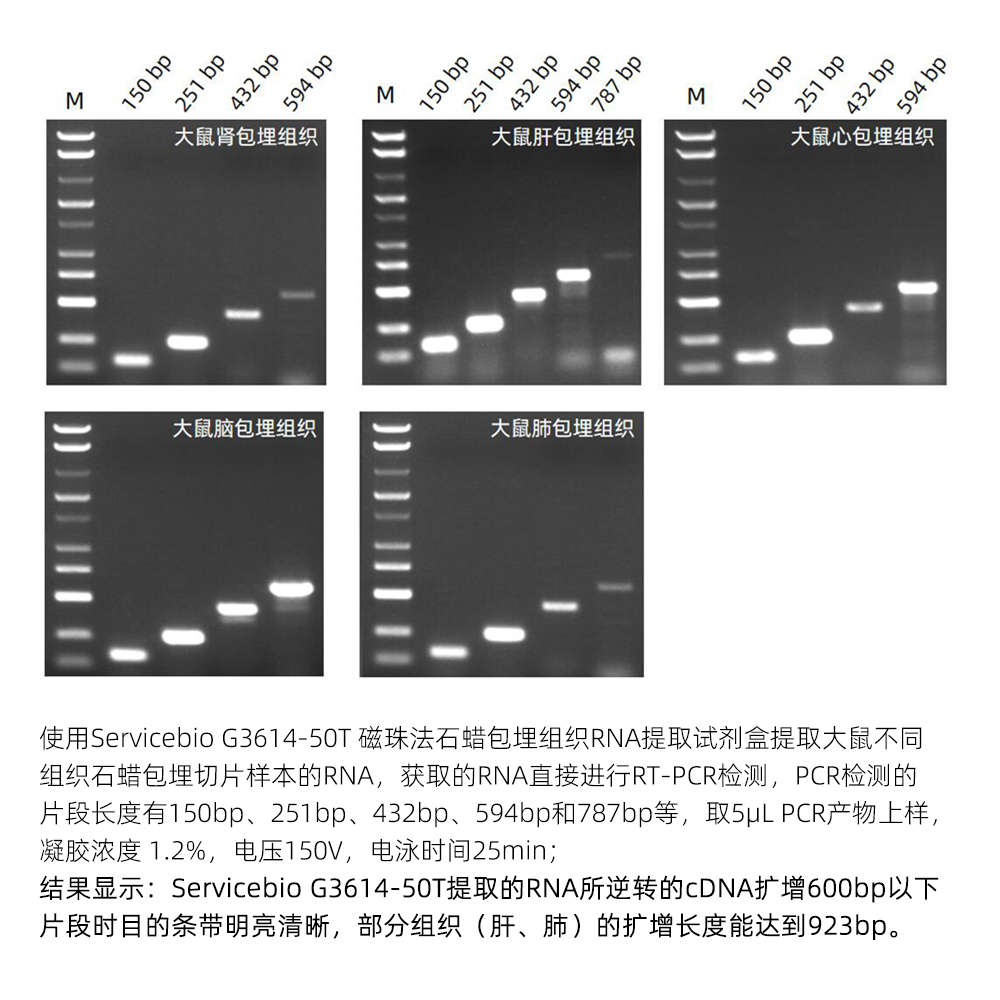
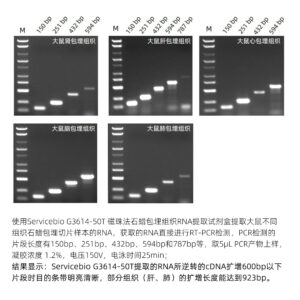
This kit is designed for RNA extraction from formalin-fixed, paraffin-embedded tissues (FFPE). It employs a special dewaxing reagent that does not contain organic solvents like xylene, making it safe and non-toxic while effectively removing paraffin and releasing tissue samples. The kit utilizes a unique lysis buffer to efficiently break down tissues and employs superparamagnetic beads for specific RNA binding, allowing for the rapid and effective extraction of RNA from tissues. The extraction process can be completed within 1 hour, resulting in a high yield of RNA with high purity and integrity. It is suitable for downstream molecular biology experiments such as RT-PCR and Real-Time PCR.
Storage and Transportation
DNase and Proteinase K are shipped on wet ice and should be stored at -20°C. All other reagents are shipped at room temperature and should be stored at room temperature. The kit has a shelf life of 12 months.
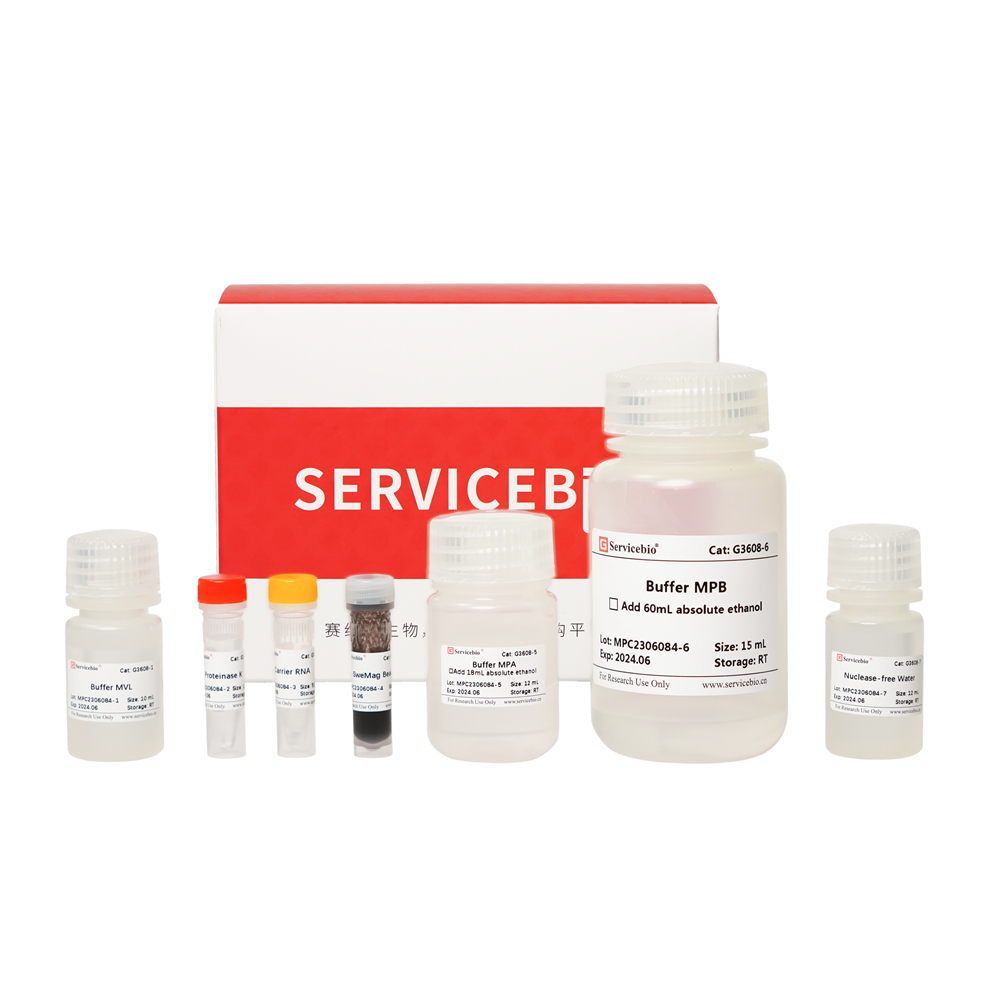
| Component Number | Component | G3614-50T |
|---|---|---|
| G3614-1 | Buffer DP | 30 mL |
| G3614-2 | Buffer FRL | 10 mL |
| G3614-3 | Proteinase K | 1 mL |
| G3614-4 | Buffer MRB | 10 mL |
| G3614-5 | SweMag Beads | 1.5 mL |
| G3614-6 | DNase | 500 μL |
| G3614-7 | 10×DNase Reaction Buffer | 1 mL |
| G3614-8 | Buffer RW1 | 8.5 mL |
| G3614-9 | Buffer RW2 | 16 mL |
| G3614-10 | Nuclease-free Water | 10 mL |
| 说明书 | Instruction Manual | 1份 |
Instructions for Use (Please read carefully before use):
- Buffer DP: If there is any precipitation, please heat and dissolve at 37°C and bring to room temperature before use.
- Before use, add DTT Solution to Buffer FRL to achieve a final concentration of 4%, i.e., add 40 µL DTT Solution to 1 mL Buffer FRL. It is recommended to prepare this lysis solution just before use. Buffer FRL with added DTT Solution can be stored at 4°C for one month.
- Before use, add 18 mL of anhydrous ethanol to Buffer RW1, and add 72 mL of anhydrous ethanol to Buffer RW2.
- Provide your own magnetic rack.
- Please refer to the instruction manual for detailed usage guidelines.
Sample Handling:
- For Paraffin Sections:
- Take 5-8 paraffin sections (5-10 μm thick, 1×1 cm² in size).
- Discard 2-3 sections if they have been exposed to air.
- For Paraffin Blocks:
- Scrape approximately 30 mg of tissue from the paraffin block.
- Remove excess paraffin and cut the sample into small pieces.
- For FFPE (Formalin-Fixed Paraffin-Embedded) Tissues:
- Blot the surface of the sample tissue with filter paper to remove excess liquid.
- Take approximately 30 mg of tissue, cut it into small pieces, and place it in a 1.5 mL centrifuge tube.
- Add 500 μL of 1× PBS buffer (pH 7.4).
- Vortex for 10 seconds and then centrifuge at 12,000 rpm at room temperature for 1 minute.
- Discard the supernatant.
- Repeat this step three times.
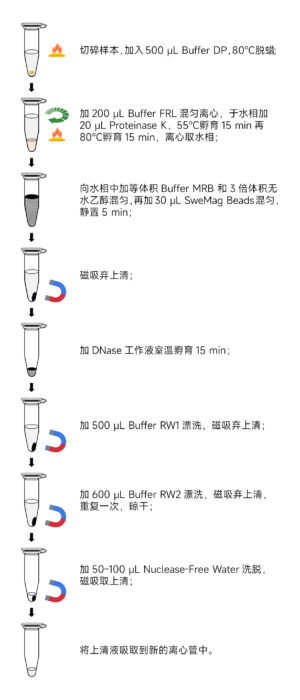
RNA Extraction Procedure:
- Transfer the prepared sample to a 1.5 mL centrifuge tube.
- Add 500 μL of Buffer DP to the tube.
- Incubate at 80°C for 3 minutes and vortex for 10 seconds while hot.
- Add 200 μL of Buffer FRL to the tube and vortex gently.
- Centrifuge at 12,000 rpm at room temperature for 1 minute.
- The solution will form two layers (an upper oil phase and a lower aqueous phase).
- Add 20 μL of Proteinase K to the aqueous phase.
- Gently pipette to mix (try not to disturb the layers).
- Incubate at 55°C for 15 minutes.
- Transfer the tube to an 80°C incubator for an additional 15 minutes.
- Invert the tube gently during this step.
- Centrifuge at 12,000 rpm at room temperature for 5 minutes.
- The solution will form two layers (an upper oil phase and a lower aqueous phase).
- Carefully transfer the lower aqueous phase to a new Nuclease-free 1.5 mL centrifuge tube, avoiding the upper oil phase and any impurities.
- Add an equal volume of Buffer MRB and three times the volume of anhydrous ethanol (precipitate may form).
- Pipette to mix.
- Add 30 μL of SweMag Beads to the tube (previously vortexed for even dispersion).
- Pipette to mix.
- Allow the tube to sit at room temperature for 5 minutes, gently pipetting 2-3 times during this period to keep the beads well dispersed.
- Place the tube on a magnetic rack for 30 seconds to allow the beads to adhere to the tube wall.
- Once the supernatant becomes clear, discard the supernatant (do not aspirate the beads).
- Remove the tube from the magnetic rack.
- Prepare the DNase working solution by pipetting 80 μL of Nuclease-free Water, 10 μL of 10× DNase Reaction Buffer, and 10 μL of DNase into a new Nuclease-free centrifuge tube.
- Gently pipette to mix.
- Add the DNase working solution to the tube containing the beads.
- Pipette to mix gently.
- Incubate at room temperature for 15 minutes, pipetting 2-3 times during this period.
- Add 500 μL of Buffer RW1 to the tube.
- Pipette to mix gently.
- Place the tube on a magnetic rack for 30 seconds to allow the beads to adhere to the tube wall.
- Once the supernatant becomes clear, discard the supernatant (do not aspirate the beads).
- Remove the tube from the magnetic rack.
- Add 600 μL of Buffer RW2 to the tube.
- Pipette to mix gently.
- Place the tube on a magnetic rack for 30 seconds to allow the beads to adhere to the tube wall.
- Once the supernatant becomes clear, discard the supernatant (do not aspirate the beads).
- Repeat step 17.
- Open the tube lid and let it sit at room temperature for 5-10 minutes to allow ethanol to evaporate (avoid excessive drying of the beads to preserve nucleic acid yield).
- Remove the tube from the magnetic rack.
- Add 50-100 μL of Nuclease-free Water to the tube.
- Pipette to mix gently.
- Let it sit at room temperature for 3 minutes.
- Place the tube on the magnetic rack until the beads are completely adhered.
- Aspirate the supernatant into a new Nuclease-free centrifuge tube to obtain high-purity RNA.
Precautions:
- Before operation, please read this product manual carefully.
- The RNA yield and integrity extracted with this kit depend on factors such as the sample type, fixation time, fixation conditions, and sample storage time. It is recommended to fix samples within 8-24 hours. If the sample fixation time exceeds 24 hours or the storage time exceeds 1 year, it may lead to significant RNA degradation.
- Ensure complete dehydration of samples before embedding to prevent residual formalin from affecting downstream experiments.
- When sampling paraffin-embedded tissues, remove excess paraffin as much as possible, and avoid exceeding 30 mg of sample quantity. Excessive sample quantity may lead to incomplete lysis, affecting nucleic acid yield.
- The magnetic beads are prone to precipitation. Shake or vortex thoroughly before use, and avoid freezing the bead suspension during storage.
- Ensure complete evaporation of ethanol before RNA elution to prevent residual ethanol from affecting downstream experiments. However, do not overdry the magnetic beads for an extended period, as it may affect RNA elution efficiency.
- Purified nucleic acids from FFPE samples are not recommended for downstream applications requiring full-length RNA.
- During the experiment, wear lab coats, disposable gloves, masks, and avoid speaking. Use Nuclease-free pipette tips and centrifuge tubes to prevent cross-contamination.
- Use a dedicated RNA extraction workbench and electrophoresis equipment.
This product is intended for research use only and is not intended for clinical diagnosis.
- For Paraffin Sections: