Description
Product Introduction
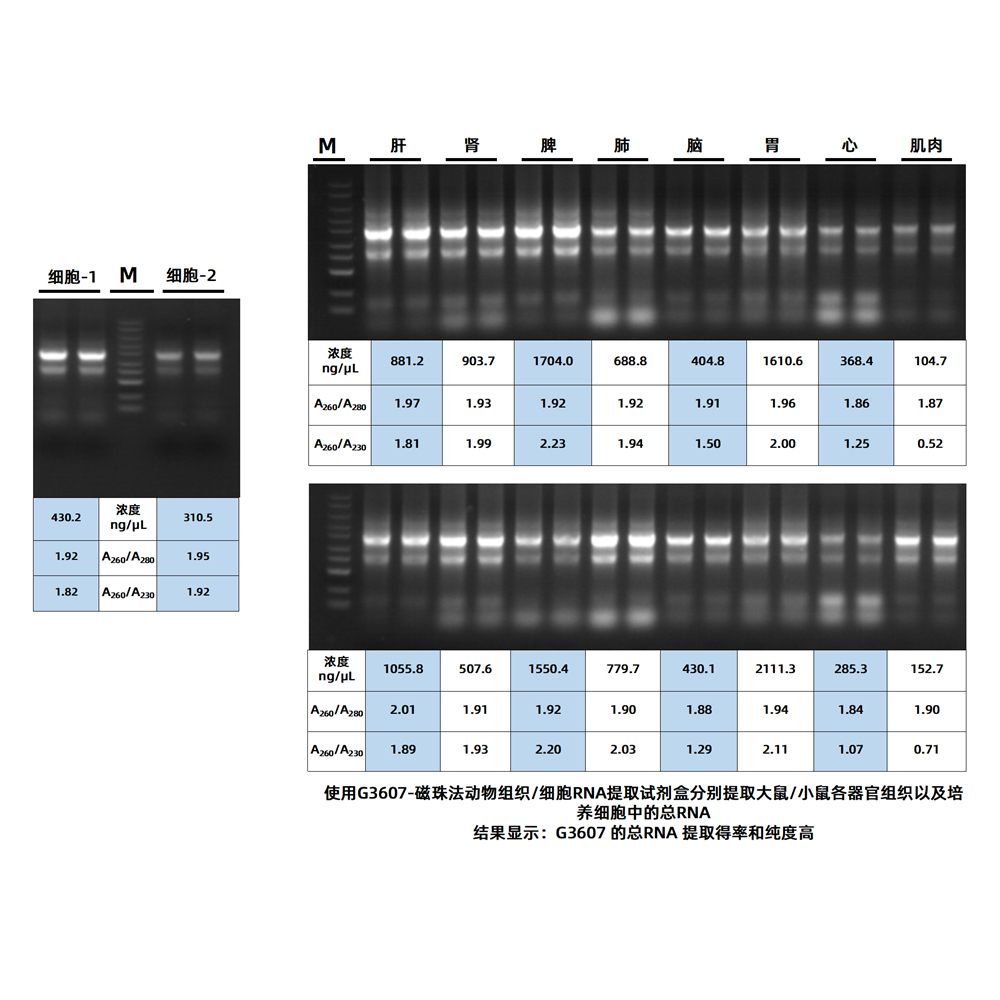
This reagent kit releases nucleic acids from animal tissues or cultured cells using a specially optimized lysis buffer. It then selectively binds total RNA from animal tissues or cultured cells using superparamagnetic beads specifically designed for RNA binding. After washing to remove residual impurities from the bead surface, high-purity RNA is eluted. This kit does not require organic reagents such as phenol or chloroform, has a short magnetic adsorption time, simple operation, and does not require multiple centrifugation steps. It can quickly extract high-purity RNA from 5-20 mg of animal tissue or fresh cultured cells (106-107). The extracted RNA can be directly used for various molecular biology experiments, including RT-PCR, RT-qPCR, Northern blotting, in vitro translation, RNase protection analysis, molecular cloning, and more.
Storage and Transportation
DNase and DTT Solution: Transported on wet ice and stored at -20°C. Other reagents can be transported and stored at room temperature. Shelf life is 12 months.
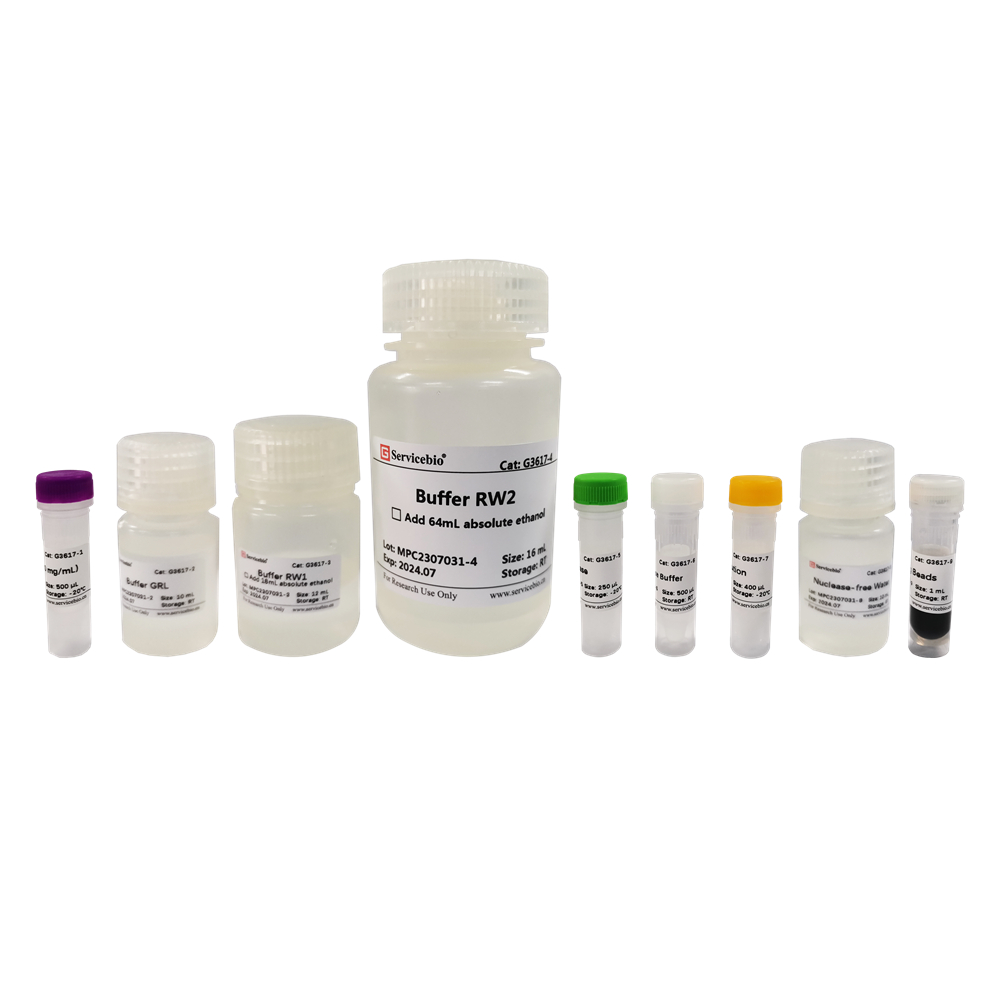
| Component Number | Component | Volume |
|---|---|---|
| G3607-50T | Buffer RL | 30 mL |
| G3607-1 | Buffer RW1 | 12 mL (add 18 mL of anhydrous ethanol before use) |
| G3607-2 | Buffer RW2 | 18 mL (add 72 mL of anhydrous ethanol before use) |
| G3607-3 | SweMag Beads | 1 mL |
| G3607-4 | DTT Solution | 1.2 mL |
| G3607-5 | DNase | 0.5 mL |
| G3607-6 | 10×DNase Buffer | 1 mL |
| G3607-7 | Nuclease-free Water | 12 mL |
| – | User Manual | 1 copy |
Preparation Before Use
- If Buffer RL exhibits any precipitation, heat it at 37°C until dissolved, and then use it once it returns to room temperature.
- Before starting, prepare Buffer RL containing 4% DTT. For every 1 mL of Buffer RL, add 40 µL of DTT Solution. This lysis buffer should be prepared immediately before use, as needed, to avoid wastage. Typically, 500 μL of Buffer RL containing DTT is needed per sample. Buffer RL with added DTT Solution can be stored at 4°C for up to one month.
- Before use, add 18 mL of anhydrous ethanol to Buffer RW1, and add 72 mL of anhydrous ethanol to Buffer RW2.
- Pre-chill the tissue homogenizer.
- Prepare a magnetic rack, isopropanol.
- Use RNase-free consumables such as centrifuge tubes and pipette tips for RNA extraction.
Operating Steps
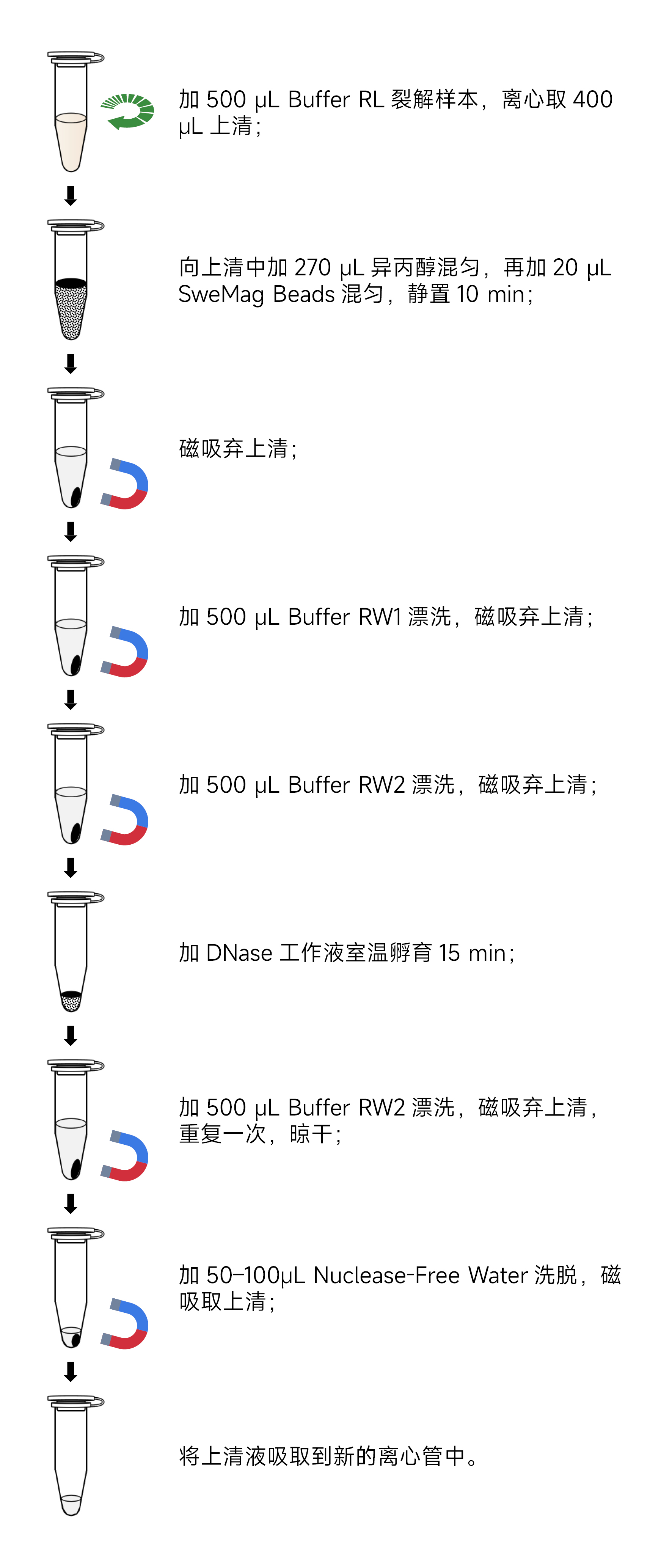
- Sample lysis:(1) For animal tissues: Take 5-20 mg of fresh, ultra-low-temperature frozen, or tissue RNA-stabilizing solution (recommended G3019) stored animal tissue. Place it in a 1.5 mL RNase-free centrifuge tube or a 2.0 mL RNase-free homogenization tube containing 2-3 3 mm grinding beads (recommended G0203-150G). Add 500 μL of Buffer RL containing 4% DTT. Use a tissue homogenizer (recommended KZ-5F-3D) to thoroughly homogenize the tissue to a homogenized state under low-temperature conditions (incomplete homogenization will affect RNA yield and quality). Incubate at room temperature for 2 min, centrifuge at 12,000 rpm for 5 min at 4°C.(2) For suspended cells: Transfer the suspended culture cells along with the culture medium to a 1.5 mL centrifuge tube. Centrifuge at 1,000 rpm for 5 min, gently remove the supernatant, and add 500 μL of Buffer RL containing 4% DTT to the cell pellet (106-107). Use a pipette to repeatedly mix until there is no obvious precipitation. Incubate at room temperature for 2 min, centrifuge at 12,000 rpm for 5 min at 4°C.(3) For adherent cells: Discard the culture medium, wash once with 1×PBS (pH 7.4), and add an appropriate amount of Buffer RL containing 4% DTT. Place the culture dish horizontally to evenly distribute the lysis solution on the cell surface and lyse the cells. Then, use a pipette to gently blow to detach the cells completely. Transfer 500 μL of the lysate containing cells to different 1.5 mL RNase-free centrifuge tubes. Incubate at room temperature for 2 min, centrifuge at 12,000 rpm for 5 min at 4°C.
- Collect 400 μL of the supernatant and transfer it to a new 1.5 mL centrifuge tube, taking care not to aspirate the pellet.
- Add 270 μL of isopropanol to the centrifuge tube, use a pipette to blow or vortex to mix well, and then add 20 μL of SweMag Beads (SweMag Beads should be shaken evenly before use). Mix well by blowing with a pipette or vortexing.
- Incubate at room temperature for 10 min. During incubation, mix several times by blowing with a pipette to keep the beads suspended.
- Place the centrifuge tube on a magnetic rack for 30 s. After the supernatant becomes clear, aspirate the supernatant.
- Add 500 μL of Buffer RW1 to the centrifuge tube, remove it from the magnetic rack, and use a pipette to blow until the beads are evenly dispersed. Place the centrifuge tube on a magnetic rack for 30 s. After the supernatant becomes clear, aspirate the supernatant.
- Add 500 μL of Buffer RW2 to the centrifuge tube, remove it from the magnetic rack, and use a pipette to blow until the beads are evenly dispersed. Place the centrifuge tube on a magnetic rack for 30 s. After the supernatant becomes clear, aspirate the supernatant.
- Remove the magnetic rack and proceed with DNase digestion:a) Prepare DNase reaction solution: Take 10 μL of 10×DNase Buffer, 10 μL of DNase, and 80 μL of Nuclease-free Water in a new 1.5 mL centrifuge tube and mix well.b) Add 100 μL of DNase reaction solution to the centrifuge tube containing the beads. Use a pipette to gently blow until the beads are evenly dispersed. Incubate at room temperature for 15 min, mixing every 5 min with a pipette.
- After digestion, add 500 μL of Buffer RW2. Use a pipette to blow or vortex until the beads are evenly dispersed. Place the centrifuge tube on a magnetic rack for 30 s. After the supernatant becomes clear, aspirate the supernatant.
- Repeat step 9.
- Leave the centrifuge tube open at room temperature for 5-10 min to allow ethanol to completely evaporate (avoid excessive drying of the beads to prevent affecting nucleic acid yield).
- Remove the magnetic rack and add 50-100 μL of Nuclease-free Water to the centrifuge tube. Use a pipette to blow gently until the beads are evenly dispersed. Incubate at room temperature for 5 min.
- Place the centrifuge tube on the magnetic rack until the beads are completely adsorbed. Aspirate the supernatant to obtain high-purity RNA.