Description
Product Introduction
The Paraffin-embedded Tissue RNA Extraction Kit is designed for extracting RNA from formalin-fixed, paraffin-embedded tissues (FFPE). It utilizes a special dewaxing reagent that does not contain organic solvents such as xylene, ensuring safety and non-toxicity while effectively removing paraffin and releasing tissue samples. The kit combines a special lysis buffer for efficient tissue lysis with a column-based centrifugation method for rapid and effective RNA extraction from paraffin-embedded tissues. The extraction process can be completed within 1 hour, yielding high RNA quantity, purity, and integrity. It is suitable for downstream molecular biology experiments such as RT-PCR and Real-Time PCR.
Storage and Transportation
DNase and Proteinase K are transported in ice packs (wet ice) and should be stored at -20°C. Other reagents can be transported and stored at room temperature. The kit has a shelf life of 12 months.
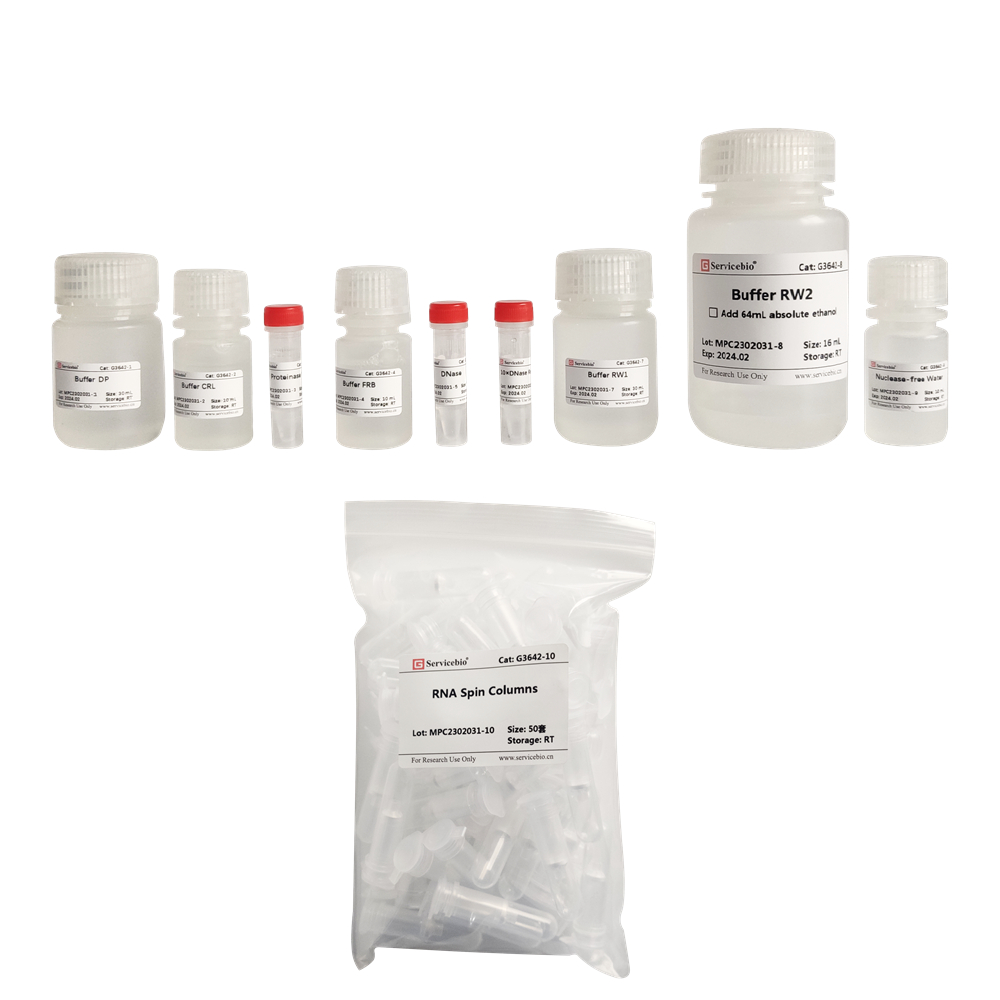
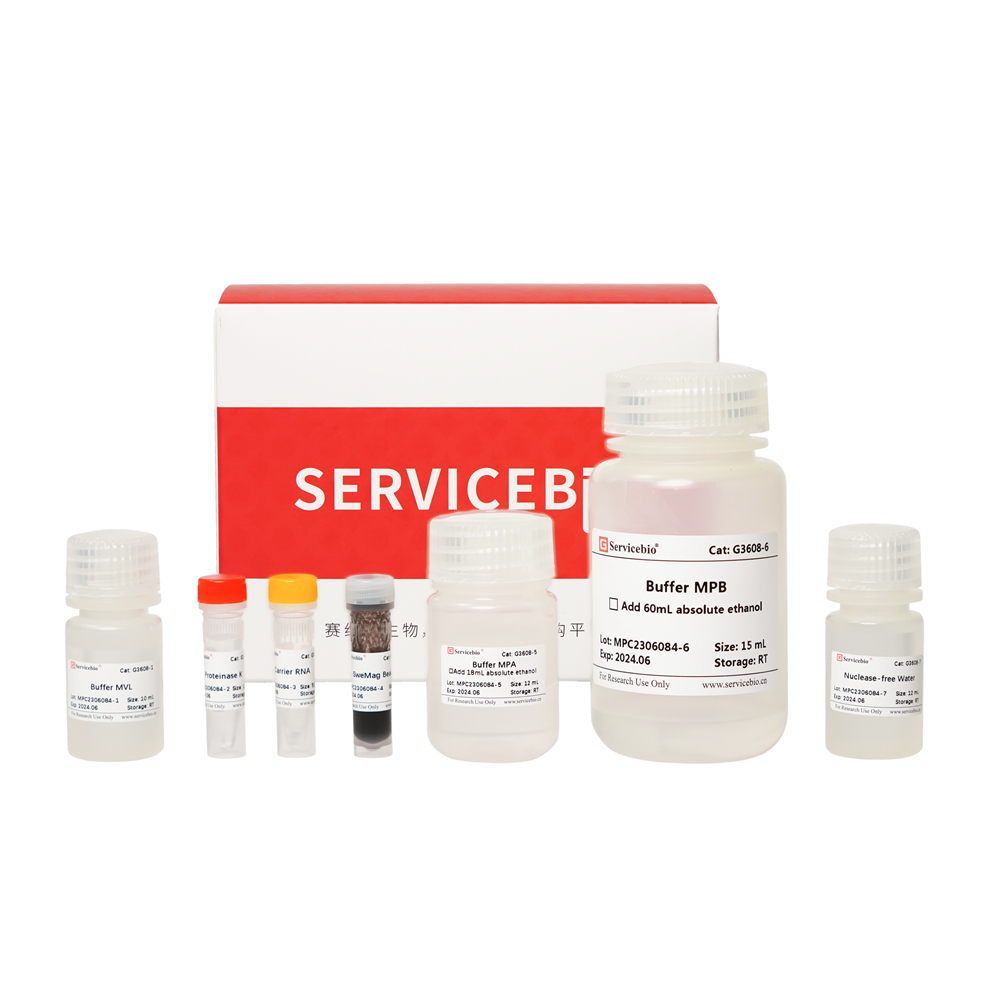
| Component Number | Component | G3642–50T |
| G3642-1 | Buffer DP | 30 mL |
| G3642-2 | Buffer CRL | 10 mL |
| G3642-3 | Proteinase K | 1 mL |
| G3642-4 | Buffer FRB | 10 mL |
| G3642-5 | DNase | 500 μL |
| G3642-6 | 10×DNase Reaction Buffer | 500 μL |
| G3642-7 | Buffer RW1 | 30 mL |
| G3642-8 | Buffer RW2 | 16 mL |
| G3642-9 | Nuclease-free Water | 10 mL |
| G3642-10 | RNA Spin Columns | 50 |
| instruction | 1 | |
Instructions for Use (Please read carefully)
- Prepare water baths or heating blocks at 80°C and 55°C in advance.
- If Buffer CRL and Buffer FRB precipitate, heat them at 65°C until dissolved. Allow them to return to room temperature before use.
- Prepare DNase working solution fresh before use.
- Prior to use, add 64 mL of anhydrous ethanol to Buffer RW2, mix well, and use.
Operating Procedure
- Sample Preparation
I. Paraffin sections: Take 5-8 paraffin sections (5-10 μm thick, 1×1 cm2 size). Discard the top 2-3 sections if exposed to air.
II. Paraffin blocks: Scrape approximately 30 mg of tissue sample from the paraffin block using a sterile surgical knife. Remove excess paraffin as much as possible and cut the sample into small pieces.
III. Formalin-fixed samples: Blot the liquid on the sample surface with filter paper. Take approximately 30 mg of sample, cut it into small pieces, and place it in a 1.5 mL centrifuge tube. Add 500 μL of 1× PBS buffer (pH 7.4) to the tube, vortex for 10 seconds, and centrifuge at 12,000 rpm at room temperature for 1 minute. Discard the supernatant and repeat this step 3 times.
- Transfer the sample to a 1.5 mL centrifuge tube. Add 500 μL of Buffer DP, incubate at 80°C for 3 minutes, and vortex for 10 seconds while hot.
- Add 200 μL of Buffer CRL to the centrifuge tube, vortex to mix, and centrifuge at 12,000 rpm at room temperature for 1 minute. The solution will separate into two layers (an upper oil phase and a lower aqueous phase).
- Add 20 μL of Proteinase K to the lower aqueous phase. Gently tap and mix with a pipette (try not to disrupt the layers) and incubate at 55°C for 15 minutes.
- Transfer the centrifuge tube to an 80°C incubator for 15 minutes (if you only have one heating module, place the tube containing the sample at room temperature and transfer it to the heating module when it reaches 80°C). Invert the tube several times to mix.
- Centrifuge at 12,000 rpm at room temperature for 5 minutes. The solution will separate into two layers (an upper oil phase and a lower aqueous phase).
- Transfer the lower aqueous phase to a new Nuclease-free 1.5 mL centrifuge tube (avoid taking the upper oil phase and other impurities). Add Buffer FRB equal in volume to the aqueous phase and three times the volume of anhydrous ethanol (precipitation may occur). Mix by pipetting.
- Transfer the entire mixture to an RNA Spin Column, centrifuge at 12,000 rpm at room temperature for 2 minutes, and discard the filtrate.
- Prepare DNase working solution: Take 35 μL of Nuclease-free Water, 5 μL of 10× DNase Reaction Buffer, and 10 μL of DNase in a new Nuclease-free centrifuge tube. Gently tap and mix with a pipette.
- Suspend the DNase working solution onto the center of the RNA Spin Column membrane. Allow it to sit at room temperature for 15 minutes.
- Add 500 μL of Buffer RW1 to the RNA Spin Column, centrifuge at 12,000 rpm at room temperature for 1 minute, and discard the filtrate.
- Add 600 μL of Buffer RW2 to the RNA Spin Column (add it along the tube wall to help flush out residual salts), centrifuge at 12,000 rpm at room temperature for 1 minute, and discard the filtrate.
- Repeat step 12.
- Place the RNA Spin Column on a collection tube, centrifuge at 12,000 rpm at room temperature for 2 minutes, and transfer the RNA Spin Column to a new Nuclease-free 1.5 mL centrifuge tube.
- Leave the RNA Spin Column uncovered at room temperature for 3-5 minutes to allow residual ethanol to evaporate completely.
- Suspend 50-100 μL of Nuclease-free Water into the center of the RNA Spin Column membrane. Allow it to sit at room temperature for 5 minutes. Centrifuge at 12,000 rpm for 2 minutes to collect the RNA solution. If a higher concentration of RNA is desired, the first eluate can be added back to the RNA Spin Column, allowed to sit at room temperature for 5 minutes, centrifuged at 12,000 rpm for 2 minutes, and eluted again.
Precautions
- Please read the product manual carefully before operation.
- The yield and integrity of RNA extracted with this kit depend on the sample type, fixation time, fixation conditions, and sample storage time. The fixation time is preferably within 8-24 hours. If the sample fixation time exceeds 24 hours or the storage time exceeds 1 year, it may result in significant RNA degradation.
- Ensure complete dehydration of the sample before embedding to prevent residual formalin from affecting subsequent experiments.
- When sampling paraffin-embedded tissues, remove excess paraffin as much as possible and cut the sample into small pieces. The sample amount should not exceed 30 mg to avoid insufficient lysis and impact on nucleic acid yield.
- Purified nucleic acids from FFPE samples are not recommended for downstream applications that require full-length RNA.
- Wear laboratory coat, disposable gloves, and mask during the experiment. Avoid talking and use Nuclease-free pipette tips and centrifuge tubes to prevent cross-contamination.
- Use a dedicated RNA extraction operation bench and electrophoresis equipment.
This product is intended for research use only and is not intended for clinical diagnosis.