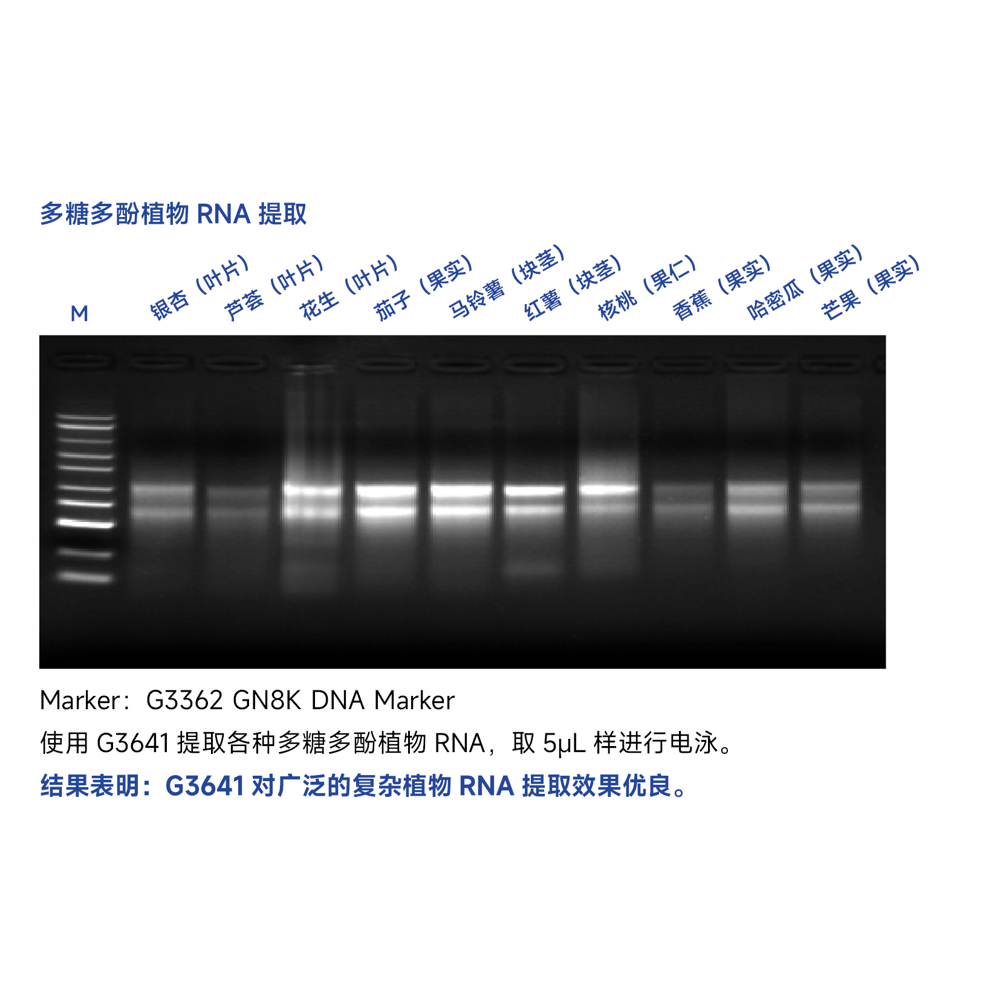
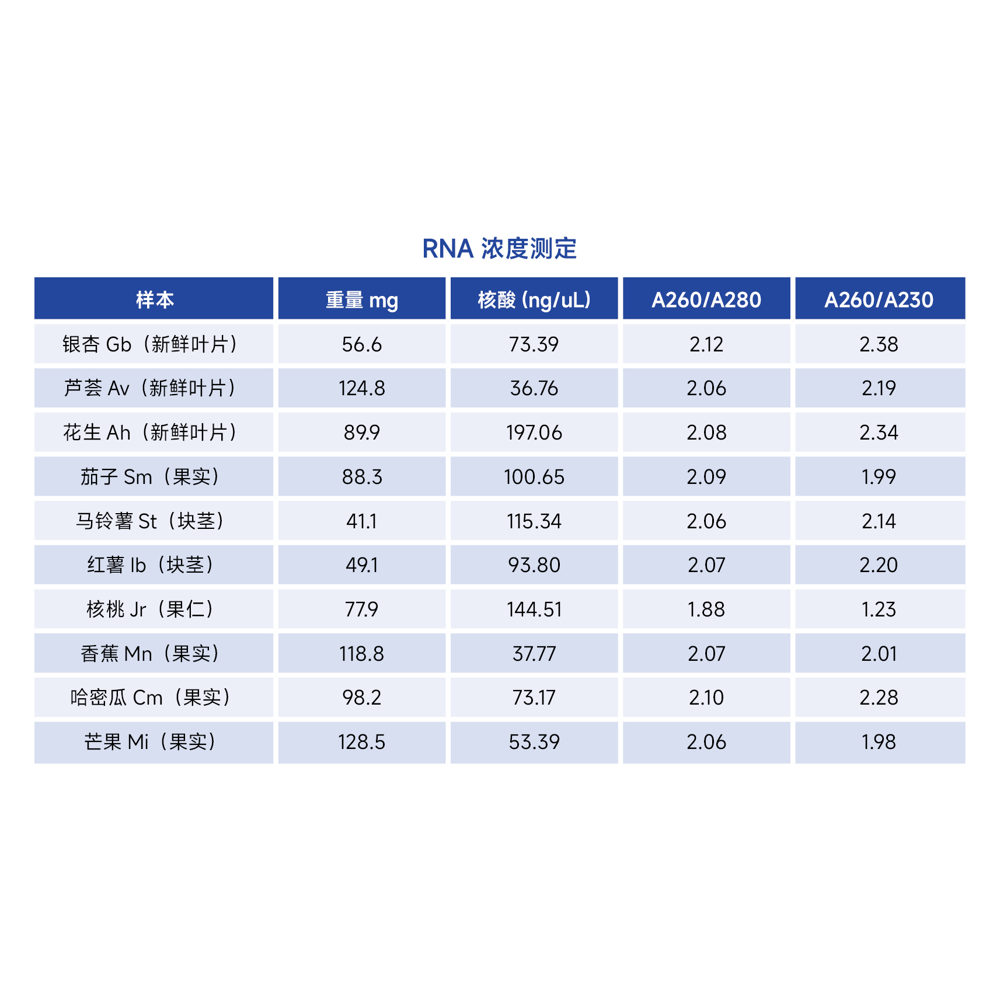
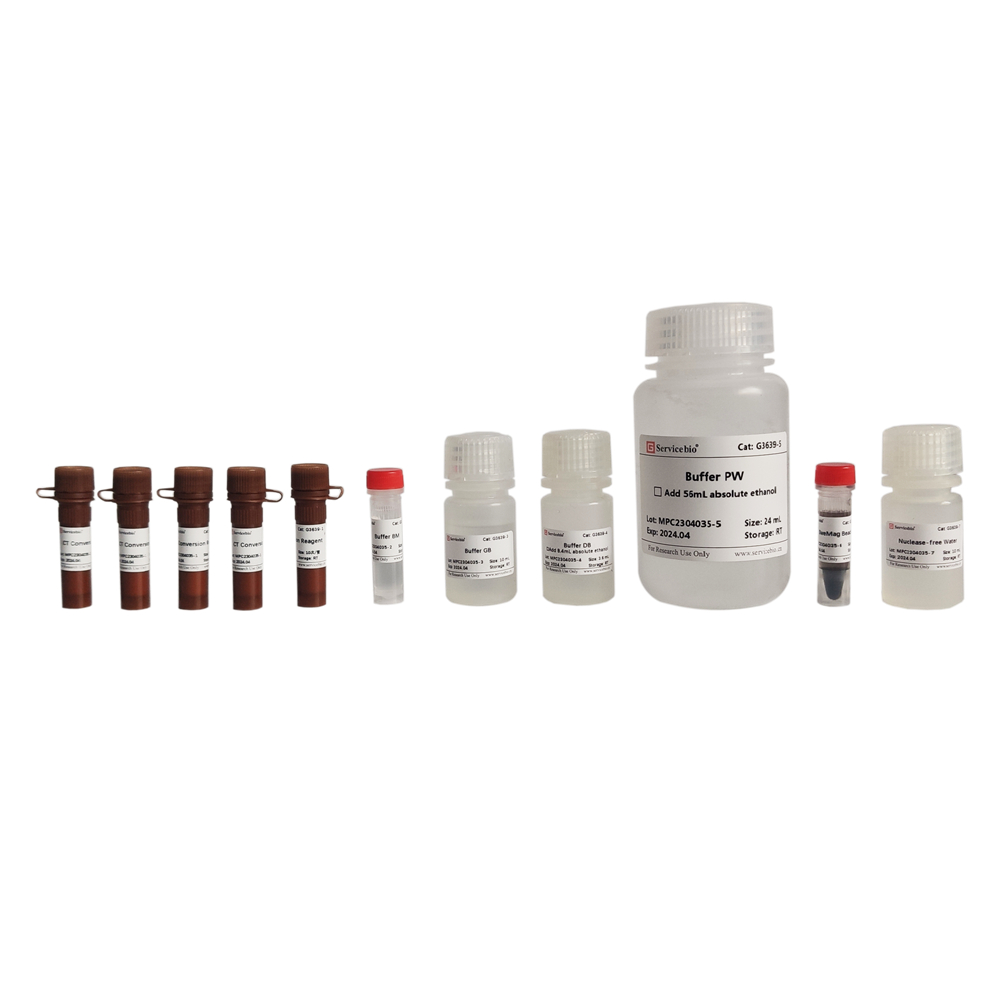
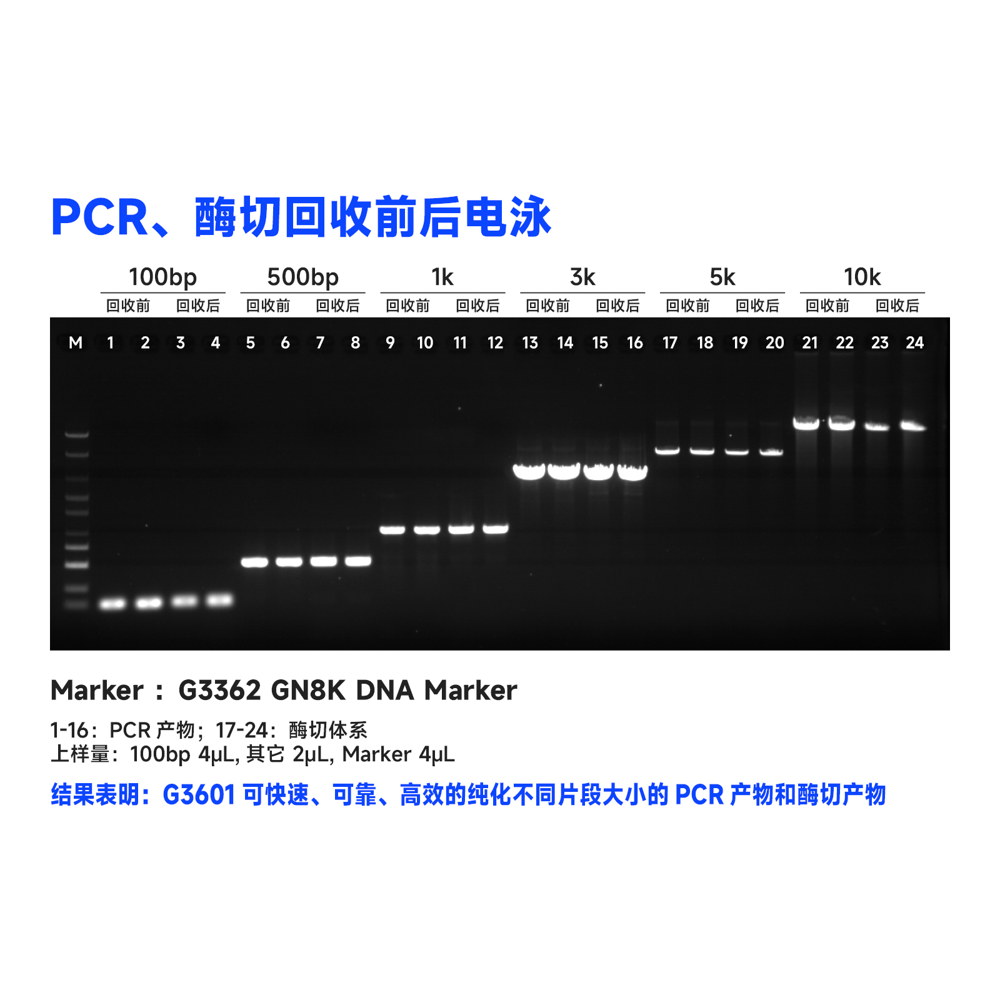
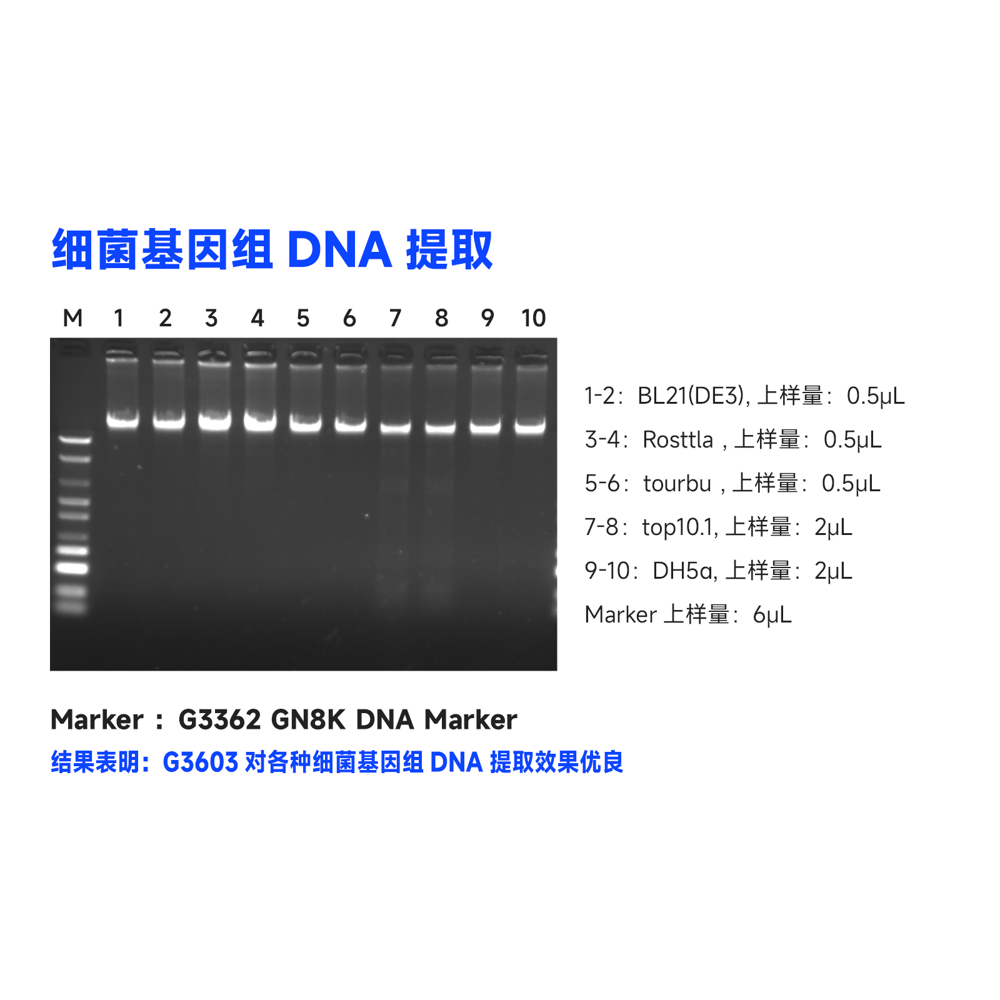
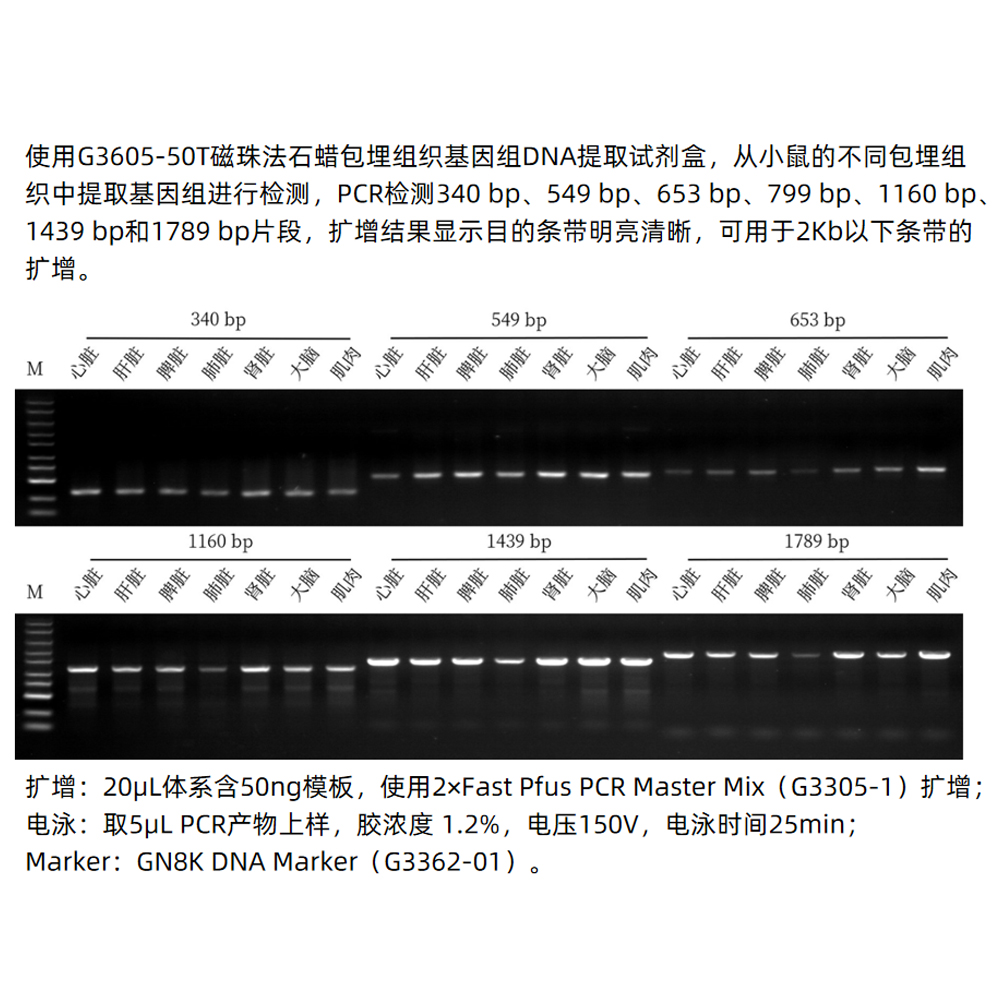
Description
Product Introduction
This kit utilizes a special lysis buffer system combined with silica membrane purification method to efficiently extract total RNA from plant tissues of less than 100 mg. It effectively removes impurities such as polysaccharides, polyphenols, and lipids from plant tissues without the need for organic reagents such as phenol or chloroform. The extracted high-purity RNA can be directly used for various molecular biology experiments, including RT-PCR, RT-qPCR, Northern blotting, cDNA library construction, microarray analysis, and more.
Storage and Transportation
DNase is shipped on wet ice and should be stored at -20°C. Other reagents can be transported and stored at room temperature. The kit has a shelf life of 12 months.
| Component Number | Component | G3641–50T |
| G3641-1 | Buffer PRL | 30 mL |
| G3641-2 | Buffer RW1 | 9 mL |
| G3641-3 | Buffer RW2 | 25 mL |
| G3641-4 | DNase | 250 μL |
| G3641-5 | 10×DNase Buffer | 500 μL |
| G3641-6 | RNase-free Water | 12 mL |
| G3641-7 | RNA Spin Columns(包含Collection Tubes) | 50 |
| Instruction | 1 | |
Precautions before Use (Please read carefully):
- Samples should be immediately extracted for RNA or stored at -80°C to prevent a decrease in the yield and quality of the extracted RNA.
- If Buffer PRL shows precipitation, heat it at 37°C and dissolve it. Use it after it has returned to room temperature.
- Before use, add β-mercaptoethanol to Buffer PRL to achieve a final concentration of 2%, i.e., add 20 µL of β-mercaptoethanol to 1 mL of Buffer PRL. It is recommended to prepare the lysis buffer just before use. After adding β-mercaptoethanol, the buffer can be stored at room temperature for one month.
- Before use, add the specified amount (indicated on the bottle) of anhydrous ethanol to Buffer RW1 and Buffer RW2.
- Pre-cool the grinder in advance when grinding the tissue using a tissue grinder.
Operating Steps:
- Sample lysis of plant tissue:
a. (Recommended) Take 50-100 mg of fresh or ultra-low-temperature frozen plant tissue and quickly transfer it to a 2.0 mL RNase-free grinding tube (recommended: HT-200-M) containing 2-3 3 mm grinding beads (recommended: G0203-150G) that have been pre-cooled with liquid nitrogen. Place the grinding tube on a tissue grinder (recommended: KZ-5F-3D) (pre-cool the adapter with liquid nitrogen before placing the grinding tube). Grind the tissue until it turns into a powder (Incomplete grinding of the sample will affect the yield and quality of RNA). Then add 500 μL of Buffer PRL and use a pipette to mix the sample and Buffer PRL thoroughly. Let it sit at room temperature for 5 minutes.
b. Take 50-100 mg of fresh or ultra-low-temperature frozen plant tissue and quickly transfer it to a 1.5 mL RNase-free centrifuge tube that has been pre-cooled with liquid nitrogen. Add liquid nitrogen and grind the tissue using a pestle, continuously adding liquid nitrogen until it turns into a powder (Incomplete grinding of the sample will affect the yield and quality of RNA). Then add 500 μL of Buffer PRL and use a pipette to mix the sample and Buffer PRL thoroughly. Let it sit at room temperature for 5 minutes.
c. Take 50-100 mg of fresh or ultra-low-temperature frozen plant tissue and transfer it to a pre-cooled mortar with liquid nitrogen. Add liquid nitrogen and grind the tissue using a pestle, continuously adding liquid nitrogen until it turns into a powder (Incomplete grinding of the sample will affect the yield and quality of RNA). Then transfer the powdered sample to a 1.5 mL RNase-free centrifuge tube containing 500 μL of Buffer PRL. Use a pipette to mix the sample and Buffer PRL thoroughly. Let it sit at room temperature for 5 minutes.
- Centrifuge at 12,000 rpm, 4°C for 5 minutes, and transfer the supernatant to a new RNase-free centrifuge tube, avoiding the sediment as much as possible.
- Add an equal volume of anhydrous ethanol to the centrifuge tube and mix by inverting.
- Transfer the above mixture to the RNA Spin Column (with a Collection Tube), adding no more than 600 µL at a time. If the volume exceeds 600 µL, add it in batches.
- Centrifuge at 12,000 rpm for 30 seconds, discard the filtrate, and place the RNA Spin Column back into the Collection Tube.
- Add 500 μL of Buffer RW1 to the RNA Spin Column, centrifuge at 12,000 rpm for 30 seconds, and discard the waste liquid.
- Add 600 μL of Buffer RW2 to the RNA Spin Column (adding Buffer RW2 along the walls of the RNA Spin Column helps to wash off residual salts on the column walls), centrifuge at 12,000 rpm for 30 seconds, and discard the waste liquid.
- Preparation of DNase reaction mixture: Take 5 µL of 10×DNase Buffer, 5 µL of DNase, and 40 µL of RNase-free Water, mix them in a new RNase-free 1.5 mL centrifuge tube.
- Add 50 μL of DNase reaction mixture to the center of the RNA Spin Column and let it stand at room temperature for 15 minutes.
- Add 500 μL of Buffer RW2 to the RNA Spin Column, centrifuge at 12,000 rpm for 30 seconds, and discard the waste liquid.
- Repeat step 10.
- Place the RNA Spin Column back into the Collection Tube and centrifuge at 12,000 rpm for 2 minutes.
- Transfer the RNA Spin Column to a new RNase-free 1.5 mL centrifuge tube and let it sit at room temperature for 3-5 minutes to allow complete evaporation of the residual ethanol in the RNA Spin Column.
- Add 50-100 μL of RNase-free Water to the center of the RNA Spin Column membrane, let it sit at room temperature for 5 minutes, centrifuge at 12,000 rpm for 2 minutes, and collect the RNA. If a higher concentration of RNA is desired, the elution liquid from the first elution step can be added back to the RNA Spin Column, let it stand at room temperature for 5 minutes, centrifuge at 12,000 rpm for 2 minutes, and collect the RNA again.
Notes:
- Wear a lab coat and disposable gloves when performing the procedure.
- Use plastic products and tips that are free of RNase to avoid cross-contamination.
- Use a dedicated RNA workstation and electrophoresis equipment. Wear a mask during the procedure to prevent contamination of utensils and reagents with RNase.
- If collecting plant samples outside the laboratory, store the samples in liquid nitrogen or dry ice to prevent a decrease in the yield and quality of the extracted RNA.
Table Attachment:
The RNA extraction yield for various plant samples using this kit is shown in the table below. The RNA yield depends on the plant species, tissue type, freshness, and growth condition. The table below is for reference only.
(Translation provided for the operating steps. The table attachment is not included.)
| Sample Types | Sample Name | RNA Yield |
|---|---|---|
| Fruits | Citrus sinensis | 1-2 μg/50 mg |
| Banana | 2-3 μg/50 mg | |
| Apple | 1-2 μg/50 mg | |
| Tubers | Potato | 10-15 μg/50 mg |
| Purple sweet potato | 4-5 μg/50 mg | |
| Seeds | Peanut | 20-25 μg/50 mg |
| Corn | 10-15 μg/50 mg |
Please note that the values provided for RNA yield are based on a 50 mg sample size. The actual RNA yield may vary depending on the specific plant species, tissue type, and other factors. This table is provided for reference purposes only.
Product is intended for research use only and is not for clinical diagnosis.
Version: V1.0-202207