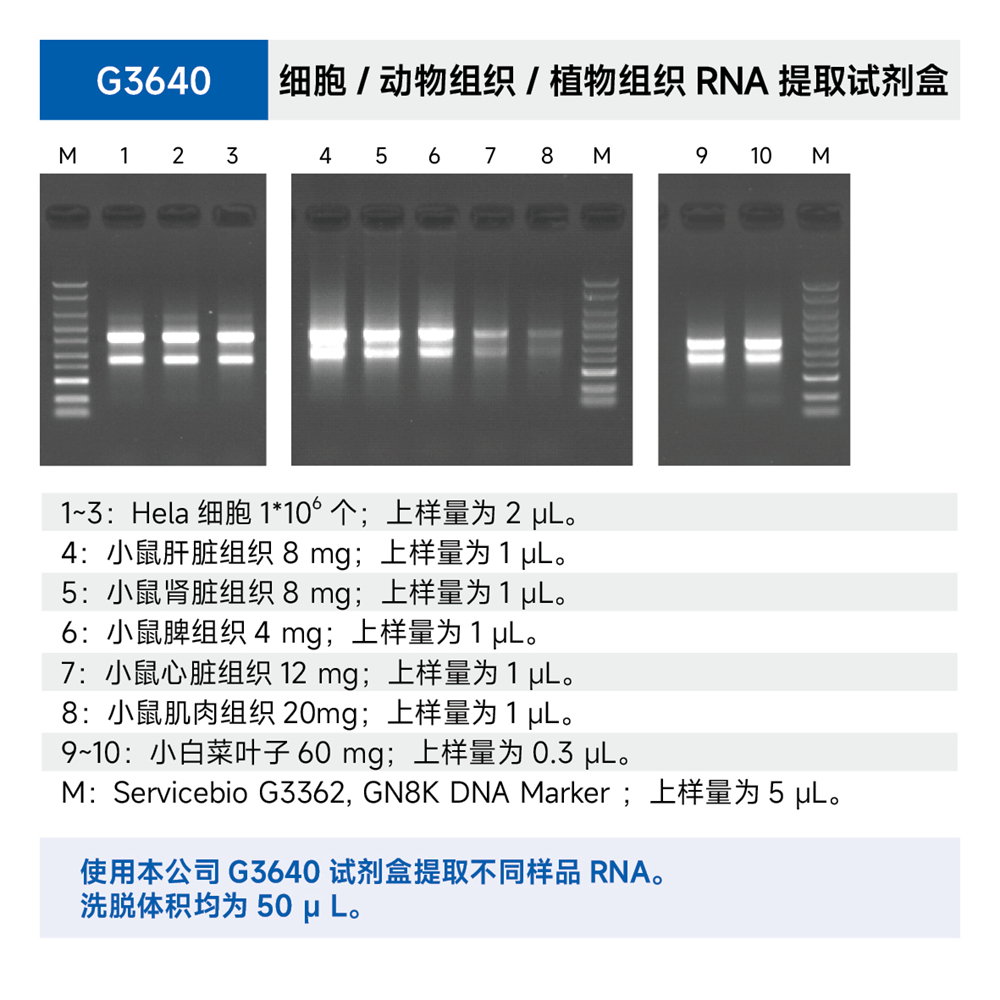
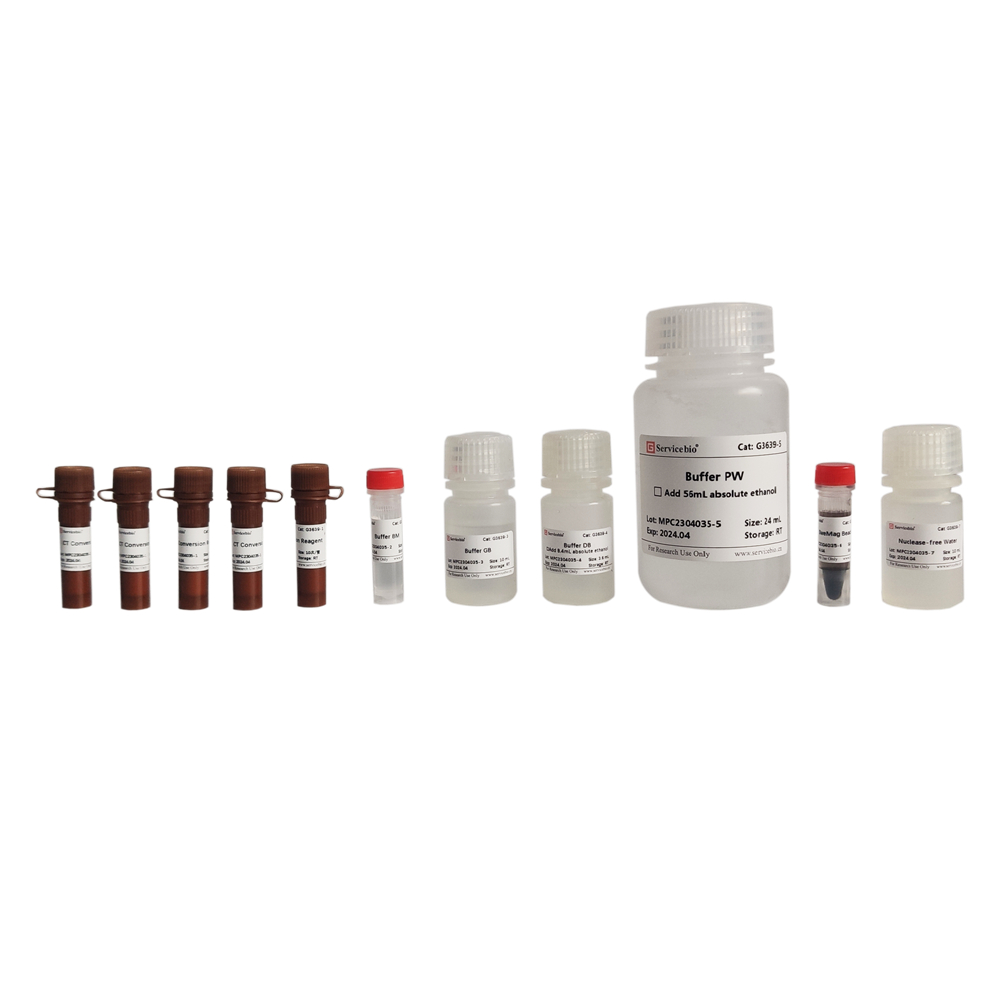
Description
To see the process in video, please click here.
Product Introduction
This kit is a versatile reagent designed for the extraction and purification of RNA from cells, animal tissues, and plant tissues (such as young leaves and stems) using a column-based method. The kit employs a special lysis system that allows the release of nucleic acids from tissues or cells through homogenization or liquid nitrogen grinding. The extraction process does not require organic reagents like phenol or chloroform. Instead, it involves the use of a gDNA Eraser Spin Column to remove genomic DNA and an RNA Spin Column to bind RNA, resulting in the extraction of high-purity RNA.
This kit offers a simple and rapid operation, enabling the extraction of high-purity RNA from 106-107 freshly cultured cells, 5-20 mg of animal tissue, and 50-100 mg of plant tissue (such as young leaves and stems) in just 30 minutes. The obtained RNA can be directly used in various molecular biology experiments, including Northern blotting, dot blotting, mRNA purification, in vitro translation, RNase protection analysis, RT-PCR, real-time RT-PCR, and cDNA library construction.
Storage and Transportation
DNase, DTT Solution: Shipped on wet ice, store at -20°C. Other reagents: Shipped at room temperature, store at room temperature. Shelf life is 12 months.
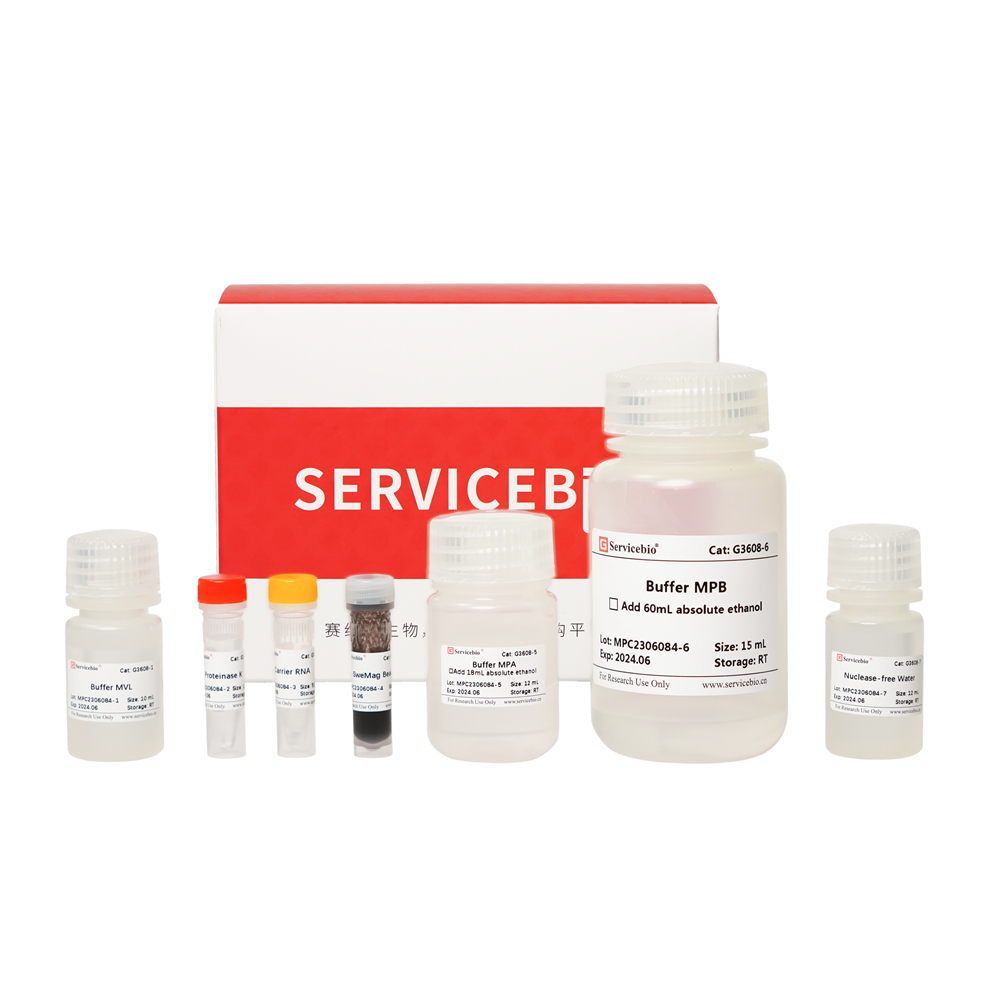
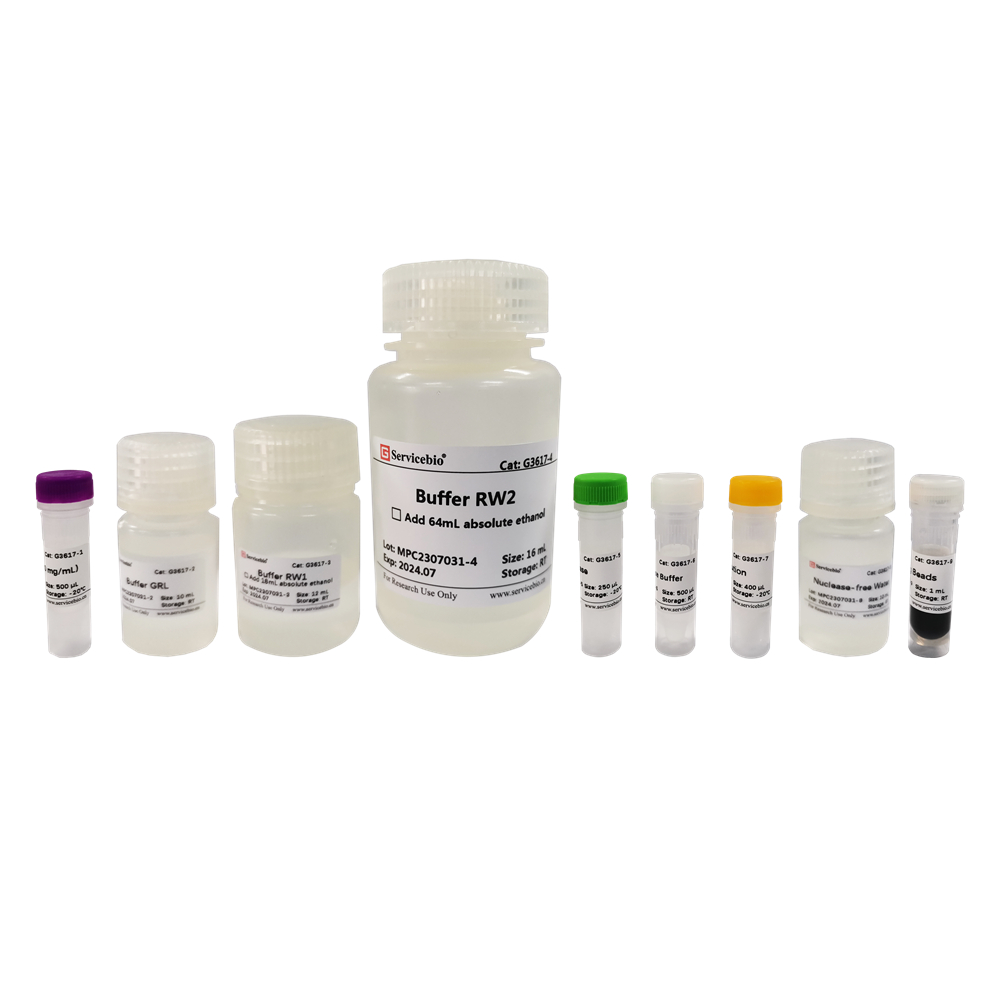
| Component Number | Component | G3640-50T |
| G3640-1 | Buffer RL1 | 30 mL |
| G3640-2 | Buffer RW1 | 12 mL (使用前加入18 mL无水乙醇) |
| G3640-3 | Buffer RW2 | 20 mL (使用前加入80 mL无水乙醇) |
| G3640-4 | DNase | 250 μL |
| G3640-5 | 10×DNase Buffer | 500 μL |
| G3640-6 | DTT Solution | 1.2 mL |
| G3640-7 | RNase-free Water | 12 mL |
| G3640-8 | gDNA Eraser Spin Columns | 50 |
| G3640-9 | RNA Spin Columns | 50 |
| instruction | 1 |
Preparation before Use
- If Buffer RL1 shows precipitation, heat and dissolve it at 37°C until it reaches room temperature before use.
- Before use, add DTT Solution to Buffer RL1 to achieve a final concentration of 4%. For 1 mL of Buffer RL1, add 40 µL of DTT Solution. It is recommended to prepare the lysis buffer fresh and use it immediately. Buffer RL1 with added DTT Solution can be stored at 4°C for one month.
- Before use, add the specified amount of absolute ethanol to Buffer RW1 and Buffer RW2 (as indicated on the bottle).
- Pre-cool the homogenizer before grinding the tissue.
Procedure
- Sample Lysis:
a) Suspension Cultured Cells: Transfer the suspension cultured cells along with the culture medium to a 1.5 mL RNase-free centrifuge tube. Centrifuge at 1,000 rpm for 5 minutes, discard the supernatant, and gently resuspend the pellet in 500 μL of Buffer RL1 (make sure DTT Solution has been added to Buffer RL1) by pipetting up and down until there are no obvious precipitates. Incubate at room temperature for 2 minutes.
b) Adherent Cultured Cells: Discard the culture medium and rinse the adherent cells once with 1× PBS (pH 7.4). Add an appropriate amount of Buffer RL1 (make sure DTT Solution has been added to Buffer RL1) to the adherent cells. Tilt the culture dish to evenly distribute the lysis buffer on the cell surface and lyse the cells. Gently pipette to detach the cells completely. Transfer 500 μL of the cell-containing lysate to separate 1.5 mL RNase-free centrifuge tubes and incubate at room temperature for 2 minutes.
c) Animal Tissue: Take 5-20 mg of fresh, ultra-low temperature frozen, or tissue stored in RNA stabilizing solution (recommended G3019) and place it in a 1.5 mL RNase-free centrifuge tube or specialized grinding tube containing 2-3 beads of 3 mm diameter (recommended G0203-150G). Immediately add 500 μL of Buffer RL1 (make sure DTT Solution has been added to Buffer RL1). Completely grind the tissue to a homogenized state using a tissue homogenizer (recommended KZ-5F-3D) under low-temperature conditions (insufficient homogenization will affect the yield and quality of RNA). Incubate at room temperature for 2 minutes, then centrifuge at 12,000 rpm, 4°C for 5 minutes.
d) Plant Tissue: Take 50-100 mg of fresh or ultra-low temperature frozen plant tissue and quickly transfer it to a pre-chilled 2.0 mL RNase-free grinding tube (recommended HT-200-M) containing 2-3 beads of 3 mm diameter. Place the grinding tube on the homogenizer (pre-chill the adapters in liquid nitrogen before placing them on the homogenizer). Grind the tissue to a powder (insufficient grinding will affect the yield and quality of RNA). Add 500 μL of Buffer RL1 and use a pipette to mix the sample thoroughly with Buffer RL1. Incubate at room temperature for 2 minutes, then centrifuge at 12,000 rpm, 4°C for 5 minutes.
- Transfer the supernatant of the cell lysate or tissue lysate from the first step to the gDNA Eraser Spin Column (avoid drawing the precipitate). Centrifuge at 12,000 rpm, room temperature for 1 minute.
- Discard the gDNA Eraser Spin Column and retain the filtrate in the Collection Tube.
- Add 2/3 volume of isopropanol to the above filtrate (precipitate may appear), and mix by pipetting.
- Immediately transfer the entire mixture (including the precipitate) to the RNA Spin Column. Add a maximum of 600 µL per round; if the volume exceeds 600 µL, add it in multiple rounds.
- Centrifuge at 12,000 rpm, room temperature for 30 seconds, discard the flow-through, and place the RNA Spin Column back into the Collection Tube.
- Add 500 μL of Buffer RW1 to the RNA Spin Column, centrifuge at 12,000 rpm, room temperature for 30 seconds, and discard the flow-through.
- Add 600 μL of Buffer RW2 to the RNA Spin Column (add Buffer RW2 along the wall of the RNA Spin Column to wash off any remaining salt on the column wall), centrifuge at 12,000 rpm, room temperature for 30 seconds, and discard the flow-through.
- DNase digestion (optional): The reagents and gDNA Eraser Spin Column in this kit can effectively remove most of the genomic DNA, and the extracted RNA contains minimal genomic DNA. For tissues with a high content of genomic DNA (such as liver, kidney, spleen) or when high RNA purity is required for subsequent experiments, DNase digestion can be performed selectively on the RNA Spin Column membrane.
a) Preparation of DNase Reaction Solution: Mix 5 µL of 10× DNase Buffer, 5 µL of DNase, and 40 µL of RNase-free Water in a new 1.5 mL RNase-free centrifuge tube.
b) Add 50 μL of DNase Reaction Solution to the center of the RNA Spin Column, incubate at room temperature for 15 minutes.
c) Add 500 μL of Buffer RW2 to the RNA Spin Column, centrifuge at 12,000 rpm, room temperature for 30 seconds, discard the flow-through, and place the spin column back into the Collection Tube.
- Repeat Step 8.
- Place the RNA Spin Column in the Collection Tube, centrifuge at 12,000 rpm, room temperature for 2 minutes to remove residual liquid.
- Place the RNA Spin Column in a new 1.5 mL RNase-free centrifuge tube and let it stand at room temperature for 3-5 minutes to allow complete evaporation of ethanol from the column.
- Add 50-100 μL of RNase-free Water to the center of the RNA Spin Column, incubate at room temperature for 5 minutes, centrifuge at 12,000 rpm, room temperature for 2 minutes to elute the RNA. If a higher concentration of RNA is desired, the first eluate can be added back to the RNA Spin Column, incubated at room temperature for 5 minutes, centrifuged at 12,000 rpm, room temperature for 2 minutes, and the RNA collected again.
Precautions
- Carefully read the instructions before performing the experiment.
- Use fresh tissue or cultured cells for extraction. If tissues or cells need to be stored for an extended period, it is recommended to use RNAsolid RNA Stabilization Solution (recommended G3019), which can quickly inhibit RNase and stabilize RNA in the samples.
- Wear lab coat, disposable gloves, and a mask during the experiment. Avoid talking and use RNase-free pipette tips and centrifuge tubes to prevent cross-contamination.
- Use a dedicated RNA extraction workstation and electrophoresis equipment.
Please note that the table provided in the appendix of the original text shows the total RNA extraction amounts for different tissues and cells. The amount of total RNA extracted from tissues or cells depends on their freshness or growth status, and the table is provided as a reference.
Sample Type Sample Name Total RNA Yield Animal Tissue Mouse Liver 30-45 μg/10 mg Animal Tissue Mouse Spleen 20-30 μg/10 mg Animal Tissue Mouse Lung 10-20 μg/10 mg Animal Tissue Mouse Kidney 20-30 μg/10 mg Animal Tissue Mouse Heart 5-10 μg/10 mg Animal Tissue Mouse Thymus 10-20 μg/10 mg Animal Tissue Mouse Brain 5-10 μg/10 mg Animal Tissue Mouse Pancreas 5-15 μg/10 mg Animal Tissue Rat Muscle 2-4 μg/10 mg Plant Tissue Cabbage Leaf 50-70 μg/100 mg Plant Tissue Corn Leaf 30-40 μg/100 mg Plant Tissue Willow Leaf 40-50 μg/100 mg Plant Tissue Green Bean Leaf 15-20 μg/100 mg Cell Hela Cells 8-15 μg/10^6 cells Please note that the values in the “Total RNA Yield” column represent the expected yield of total RNA from the specified amount of sample. The units for total RNA yield are micrograms (μg) per milligram (mg) of tissue or micrograms per 100 milligrams (mg) of plant tissue. For cell samples, the yield is expressed as micrograms per million (10^6) cells.