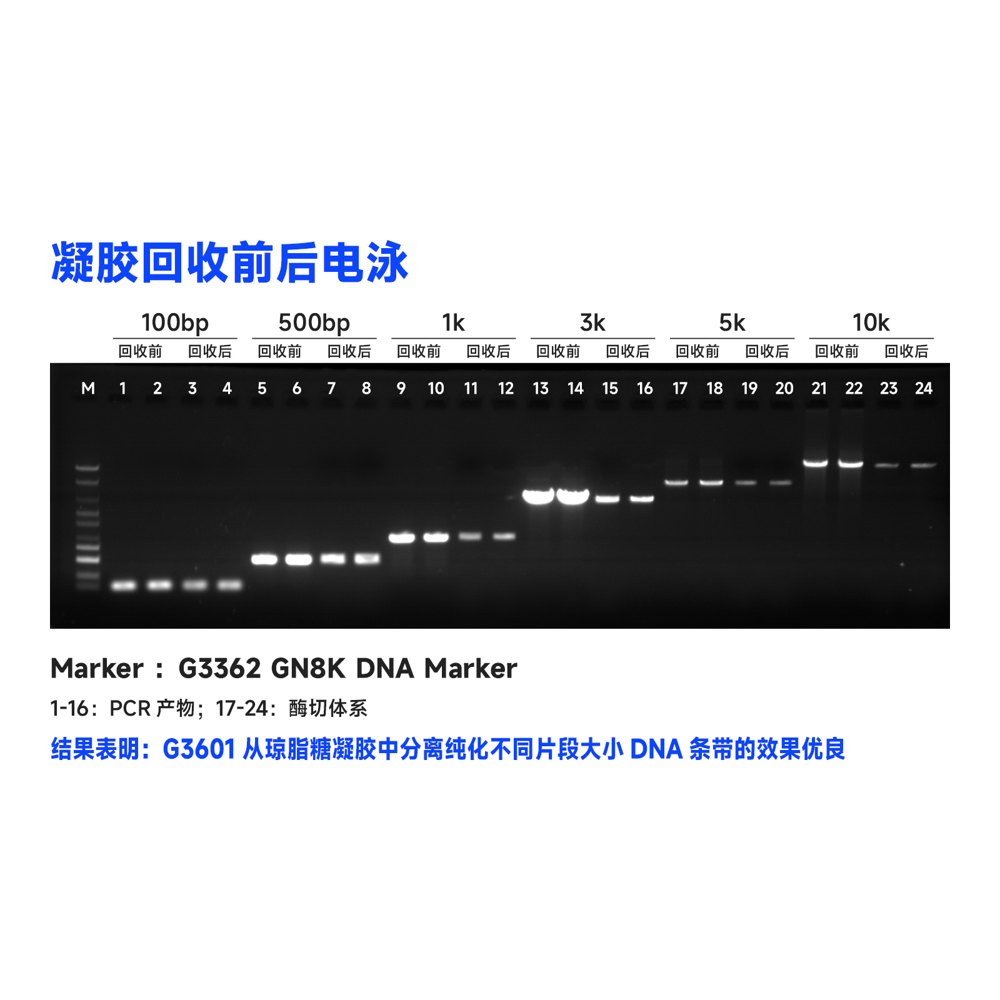
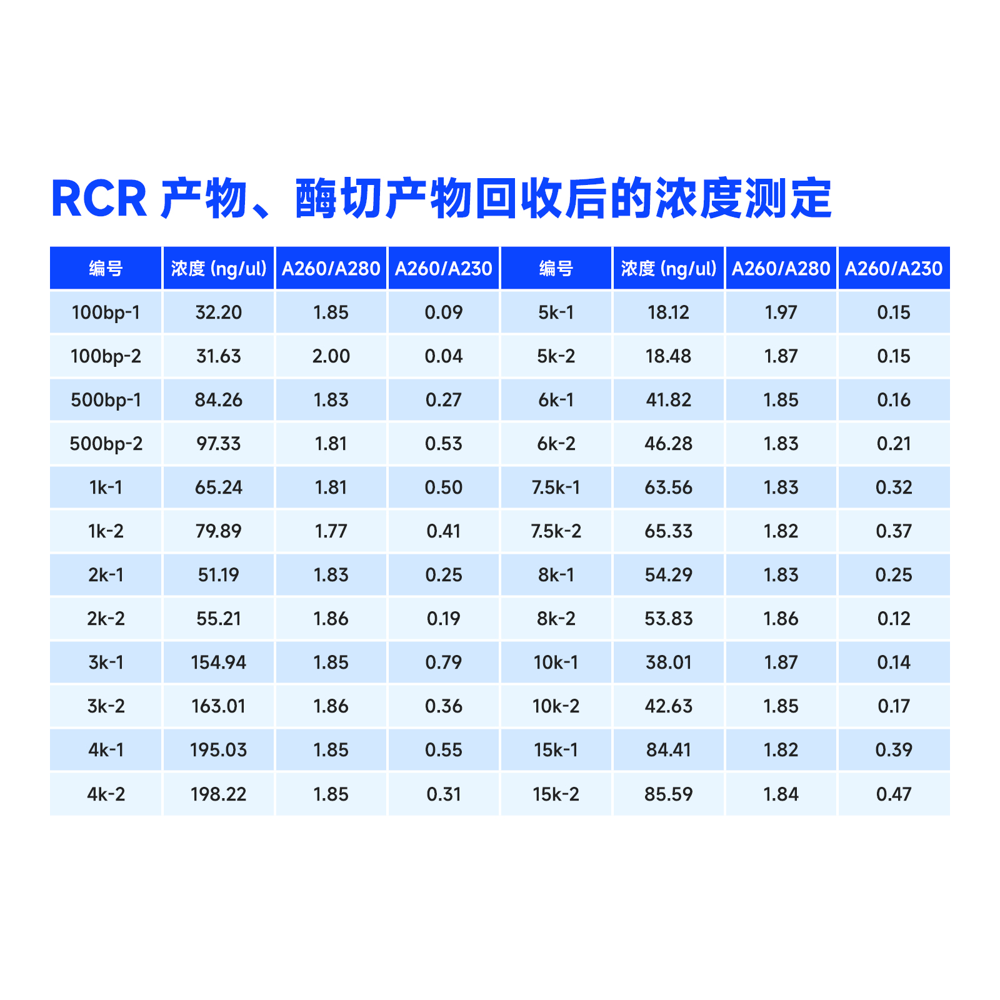
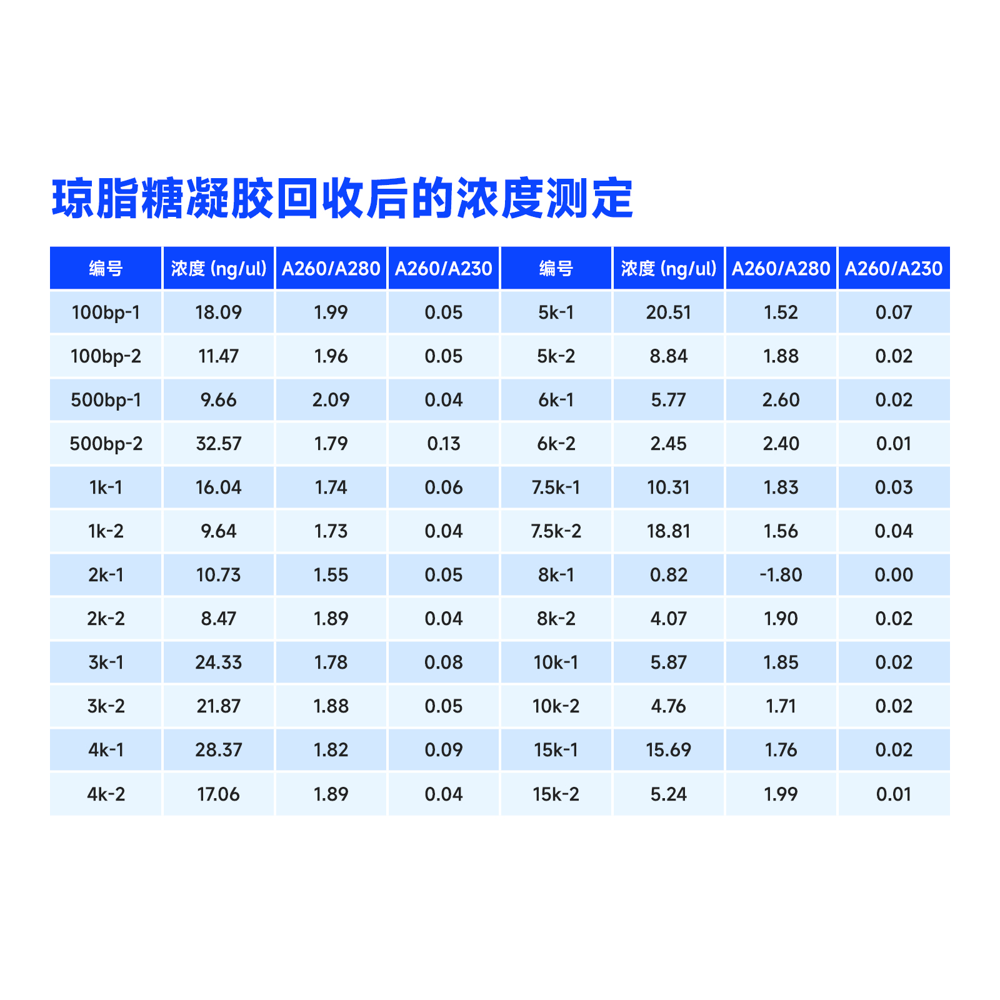
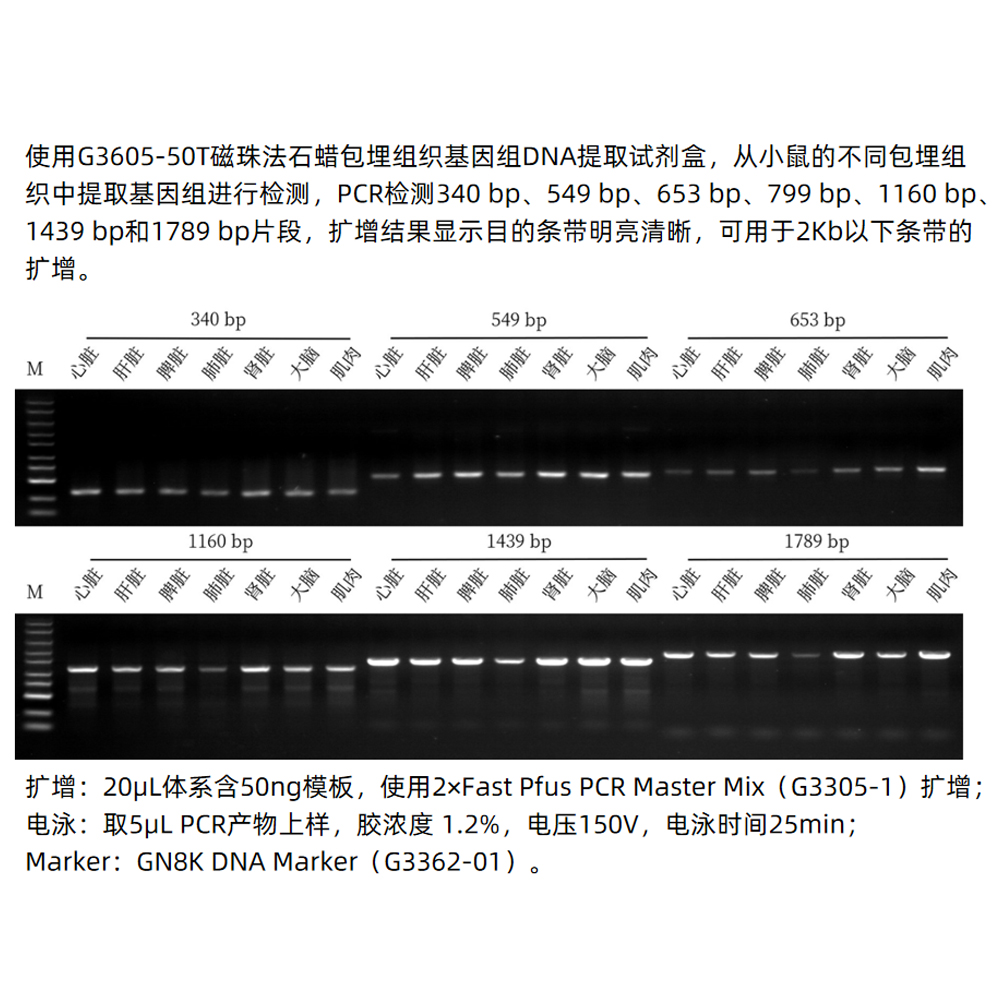
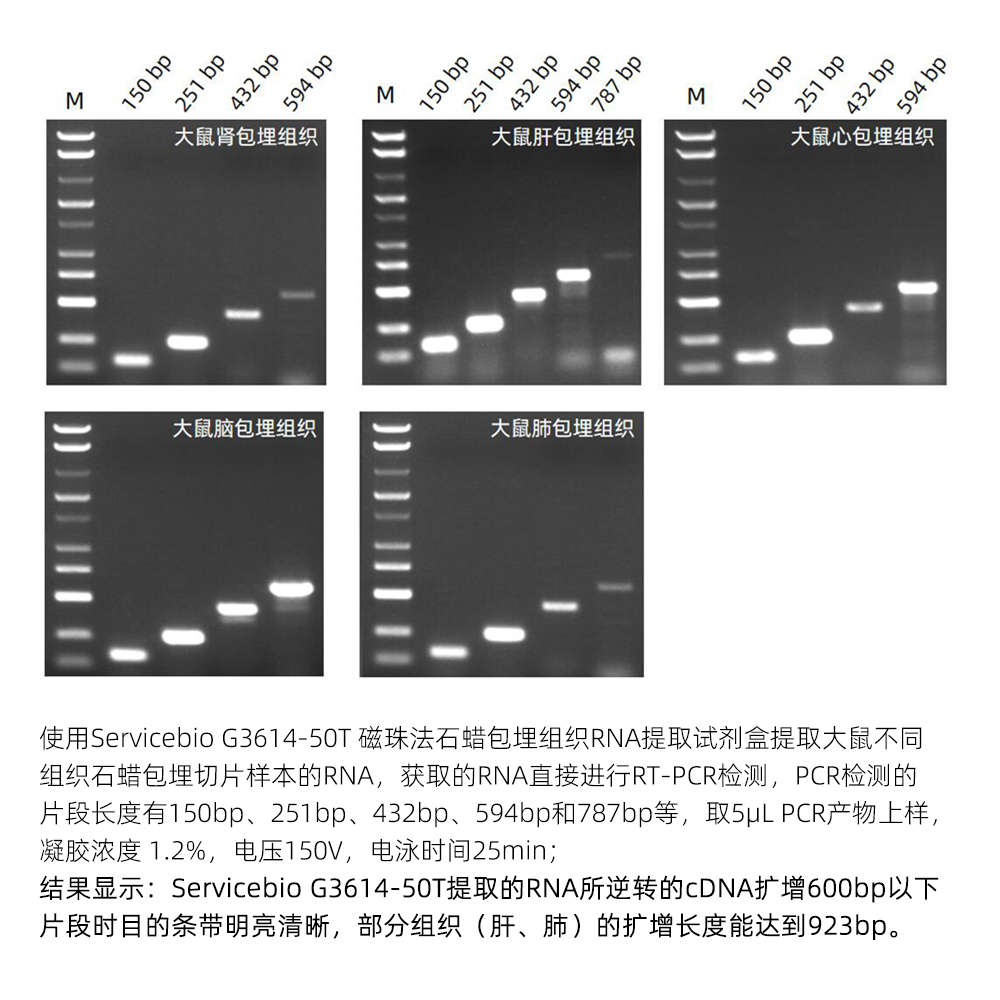
Description
- Product Name: Universal Magnetic Bead DNA Purification Kit
- Product Number: G3601-100T
- Specifications: 100T
Product Introduction
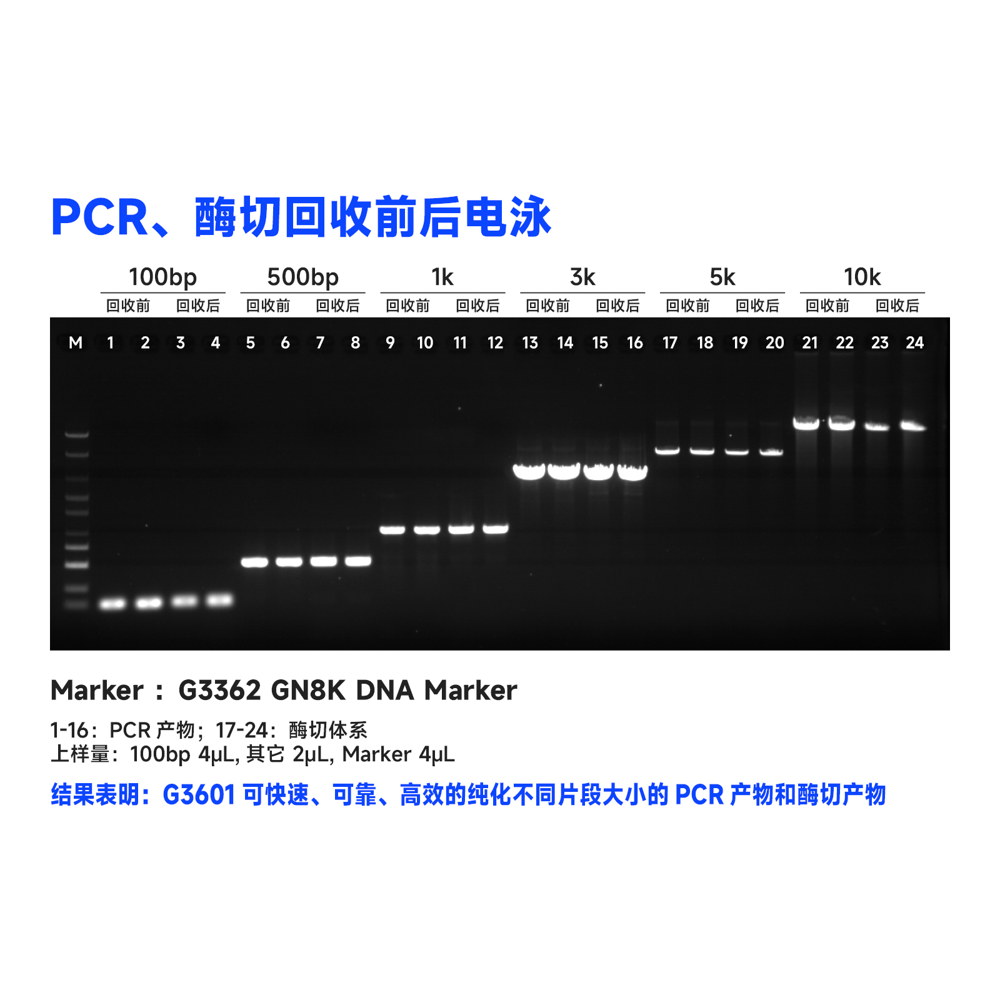
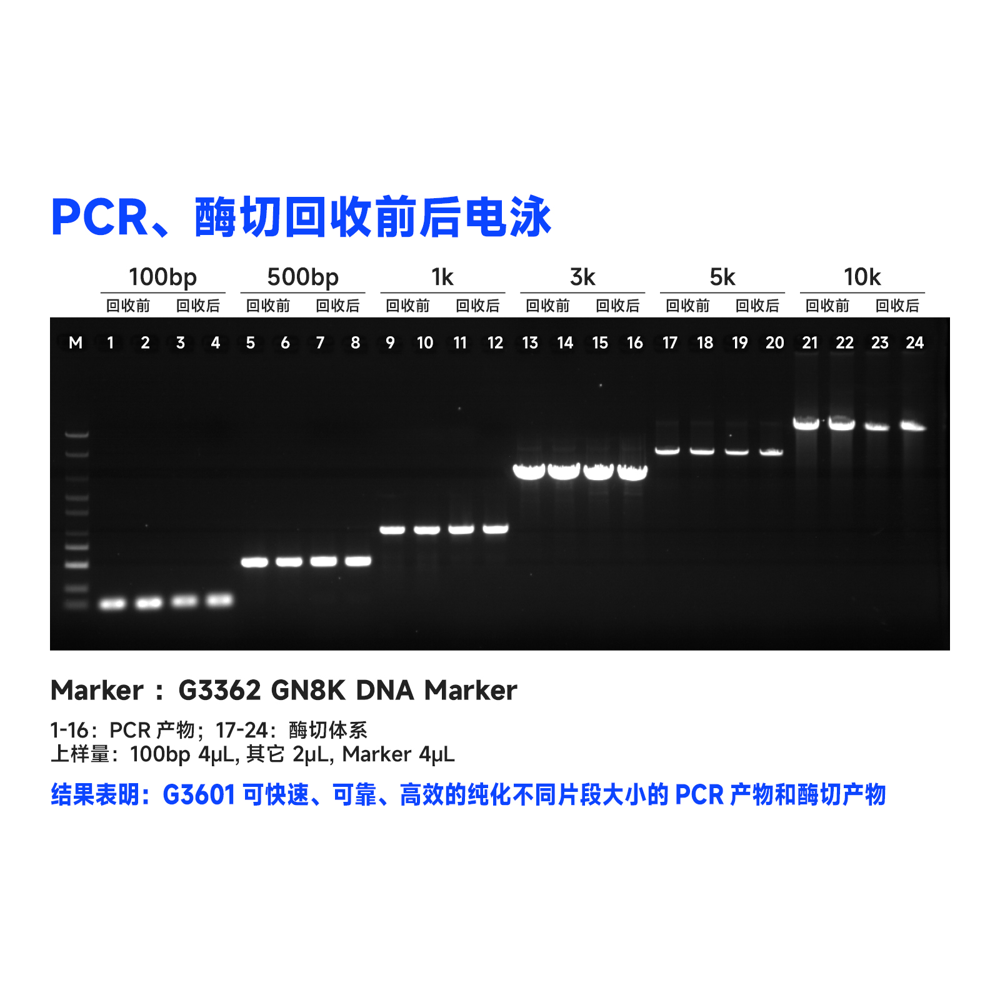
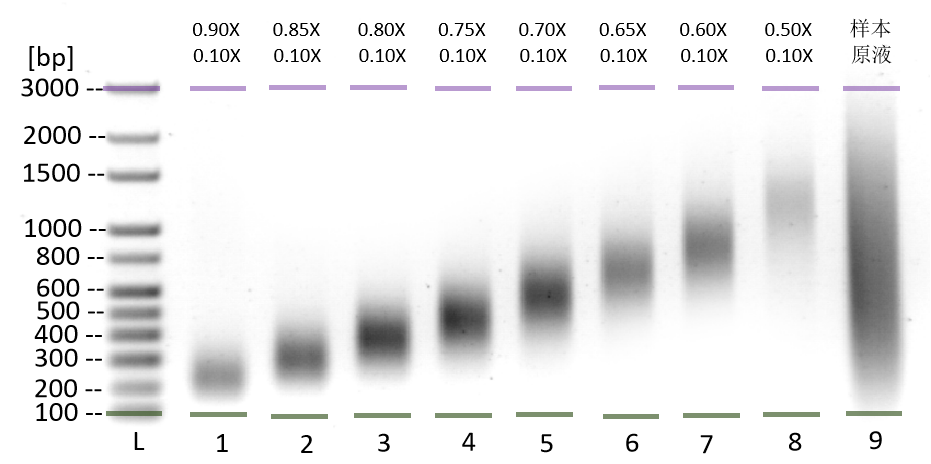
This kit utilizes the reversible binding property of magnetic beads to nucleic acids and a unique buffer system for the rapid and efficient purification of PCR products and restriction enzyme digests. It can also isolate high-purity specific DNA fragments from agarose gels. The entire procedure does not require centrifugation, and more than 80% of DNA fragments ranging from 100 bp to 10 kb can be recovered in just 30 minutes. Purified DNA fragments can be used in molecular biology experiments such as restriction enzyme reactions, ligation reactions, PCR amplification, and sequencing.
Storage and Transportation
Shipped and stored at room temperature; shelf life of 12 months.
Components
| Component Number | Component | G3601-100T |
| G3601-1 | Buffer SPS | 50 mL |
| G3601-2 | SweMag Beads | 6 mL |
| G3601-3 | Buffer SPW | 24 mL |
| G3601-4 | Buffer TE | 10 mL |
| 说明书 | 1份 | |
- tube, and weigh it.
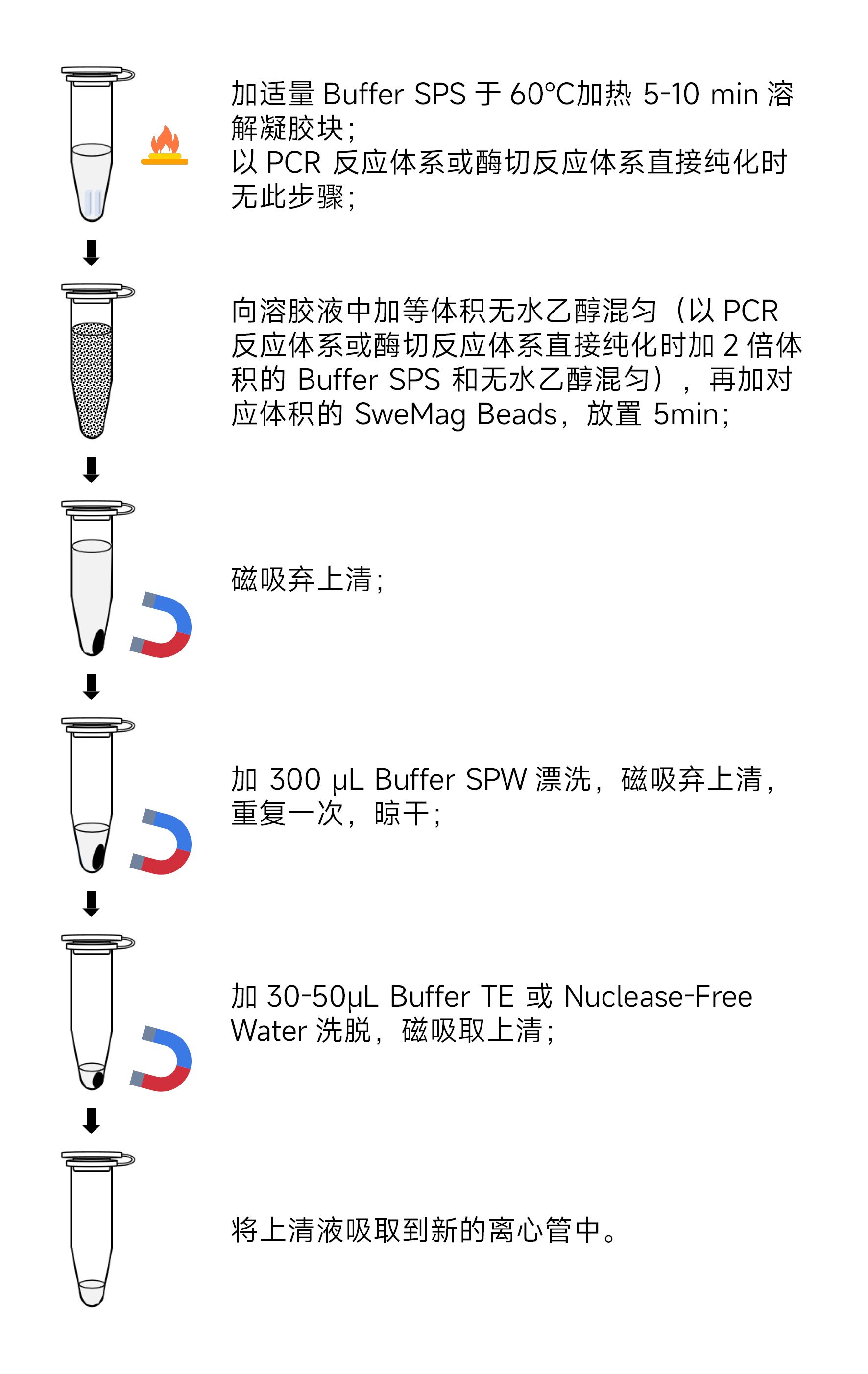
- Add a certain amount of Buffer SPS to the centrifuge tube (e.g., if the weight of the gel slice is 0.1 g, add 100 µL of Buffer SPS). Heat at 60°C for 5-10 minutes, continuously invert the tube during this period until the gel slice is completely dissolved.
- Add an equal volume of ethanol to the solution obtained in the previous step, mix gently using a pipette or vortex, then add 0.6 times the volume of PCR or digestion volume of SweMag Beads (vortex the SweMag Beads before use to ensure even distribution). Mix gently.
- Leave it at room temperature for 5 minutes, during which mix 2-3 times using a pipette or vortex until the magnetic beads are evenly dispersed.
- Place the centrifuge tube on the magnetic rack for 30 seconds or until the magnetic beads are completely adsorbed. Invert the tube gently a few times to wash off magnetic beads adhering to the tube walls. Once the supernatant is clear, discard it. (Do not aspirate the magnetic beads to avoid affecting the extraction efficiency.)
- Add 300 μL Buffer SPW to the tube. Remove from the magnetic rack and gently pipette until the magnetic beads are evenly dispersed.
- Place the centrifuge tube on the magnetic rack for 30 seconds or until the magnetic beads are completely adsorbed. Invert the tube gently a few times to wash off magnetic beads adhering to the tube walls. Once the supernatant is clear, discard it. (Be sure to aspirate all residual liquid from the centrifuge tube to avoid affecting extraction efficiency.)
- Repeat step 7.
- Remove the tube cap and leave it at room temperature for 5-10 minutes or at 65°C for 3-5 minutes to allow ethanol to completely evaporate (avoid over-drying the magnetic beads, as it may affect nucleic acid yield).
- Remove from the magnetic rack and add 30-50 μL Buffer TE or Nuclease-free Water. Pipette gently to evenly disperse the magnetic beads. Allow it to sit at room temperature for 3-5 minutes.
- Place the centrifuge tube on the magnetic rack until the magnetic beads are fully adsorbed. Aspirate the supernatant into a new centrifuge tube to obtain high-purity DNA.
Procedure for Recovering DNA from PCR or Digestion Reactions
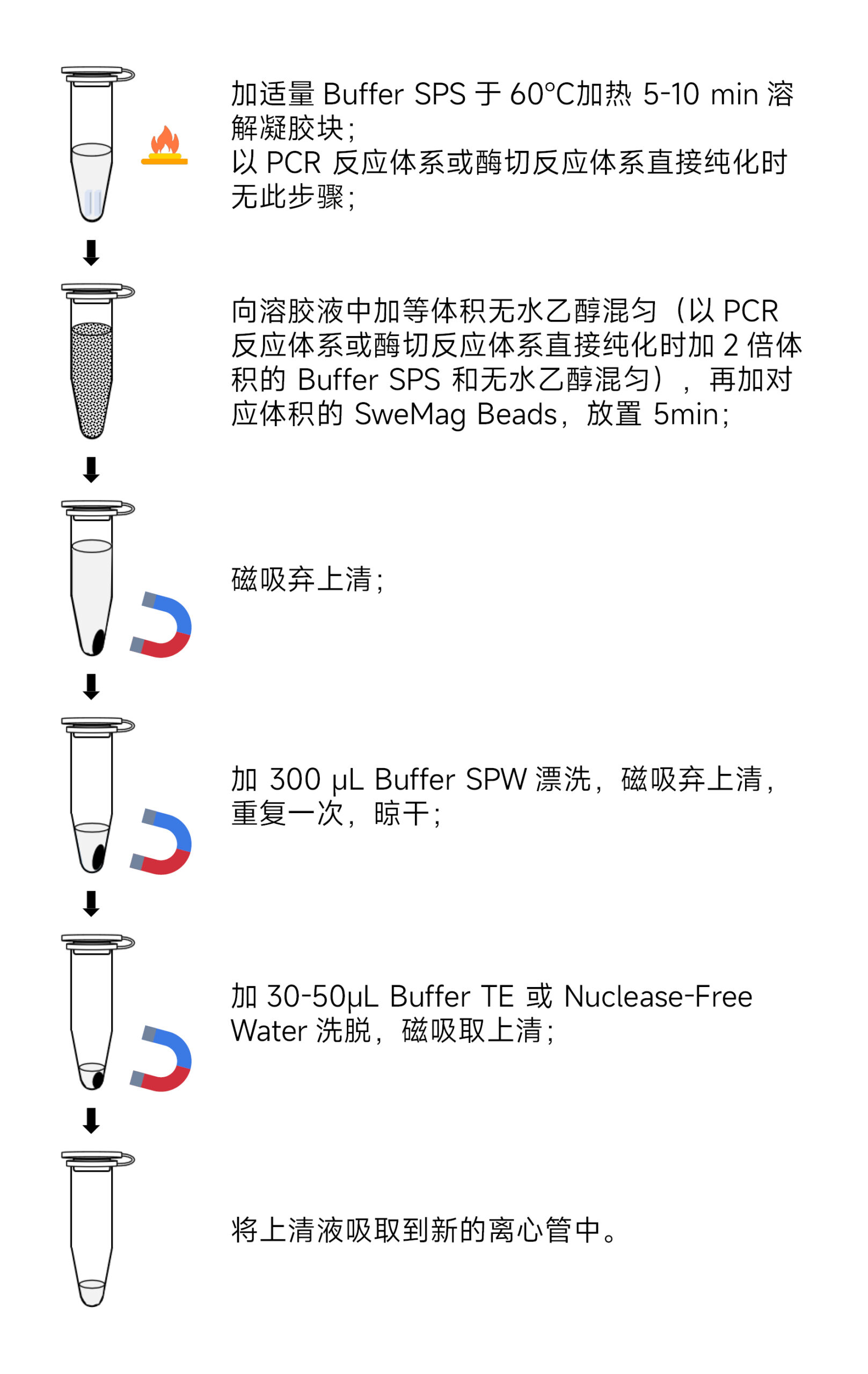
- Add twice the volume of Buffer SPS and ethanol to the PCR or digestion reaction mixture. Mix gently using a pipette or vortex, then add 0.6 times the volume of the PCR or digestion reaction volume of SweMag Beads (vortex the SweMag Beads before use to ensure even distribution). Mix gently.
- Leave it at room temperature for 5 minutes, during which mix 2-3 times using a pipette or vortex until the magnetic beads are evenly dispersed.
- Place the centrifuge tube on the magnetic rack for 30 seconds or until the magnetic beads are completely adsorbed. Invert the tube gently a few times to wash off magnetic beads adhering to the tube walls. Once the supernatant is clear, discard it. (Do not aspirate the magnetic beads to avoid affecting the extraction efficiency.)
- Add 300 μL Buffer SPW to the tube. Remove from the magnetic rack and gently pipette until the magnetic beads are evenly dispersed.
- Place the centrifuge tube on the magnetic rack for 30 seconds or until the magnetic beads are completely adsorbed. Invert the tube gently a few times to wash off magnetic beads adhering to the tube walls. Once the supernatant is clear, discard it. (Be sure to aspirate all residual liquid from the centrifuge tube to avoid affecting extraction efficiency.)
- Repeat step 5.
- Remove the tube cap and leave it at room temperature for 5-10 minutes or at 65°C for 3-5 minutes to allow ethanol to completely evaporate (avoid over-drying the magnetic beads, as it may affect nucleic acid yield).
- Remove from the magnetic rack and add 30-50 μL Buffer TE or Nuclease-free Water. Pipette gently to evenly disperse the magnetic beads. Allow it to sit at room temperature for 3-5 minutes.
- Place the centrifuge tube on the magnetic rack until the magnetic beads are fully adsorbed. Aspirate the supernatant into a new centrifuge tube to obtain high-purity DNA