Description
Product Information
| Product Name | Cat.No. | Spec. |
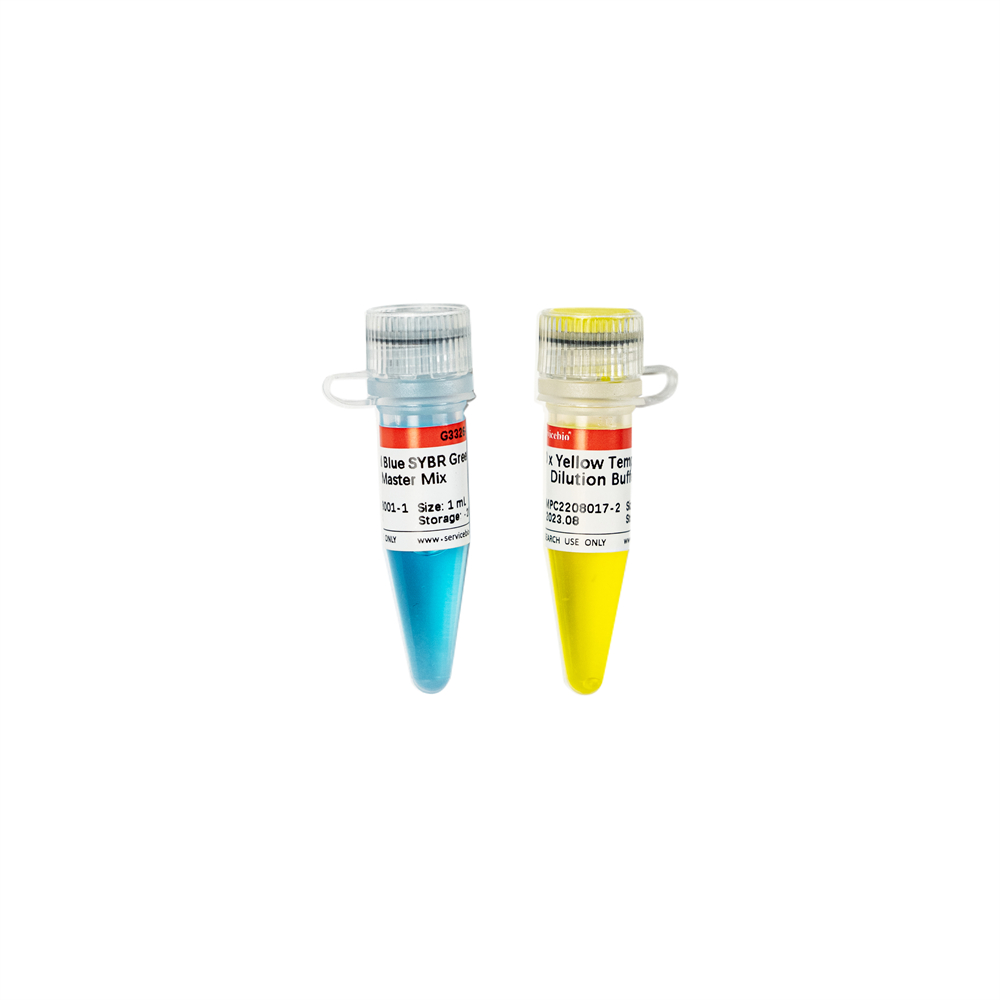
| 2×Universal Blue SYBR Green qPCR Master Mix With 40×Yellow Template Dilution Buffer | G3326-01 | 1 mL |
| G3326-05 | 5×1 mL | |
| G3326-15 | 15×1 mL |
Description/Introduction
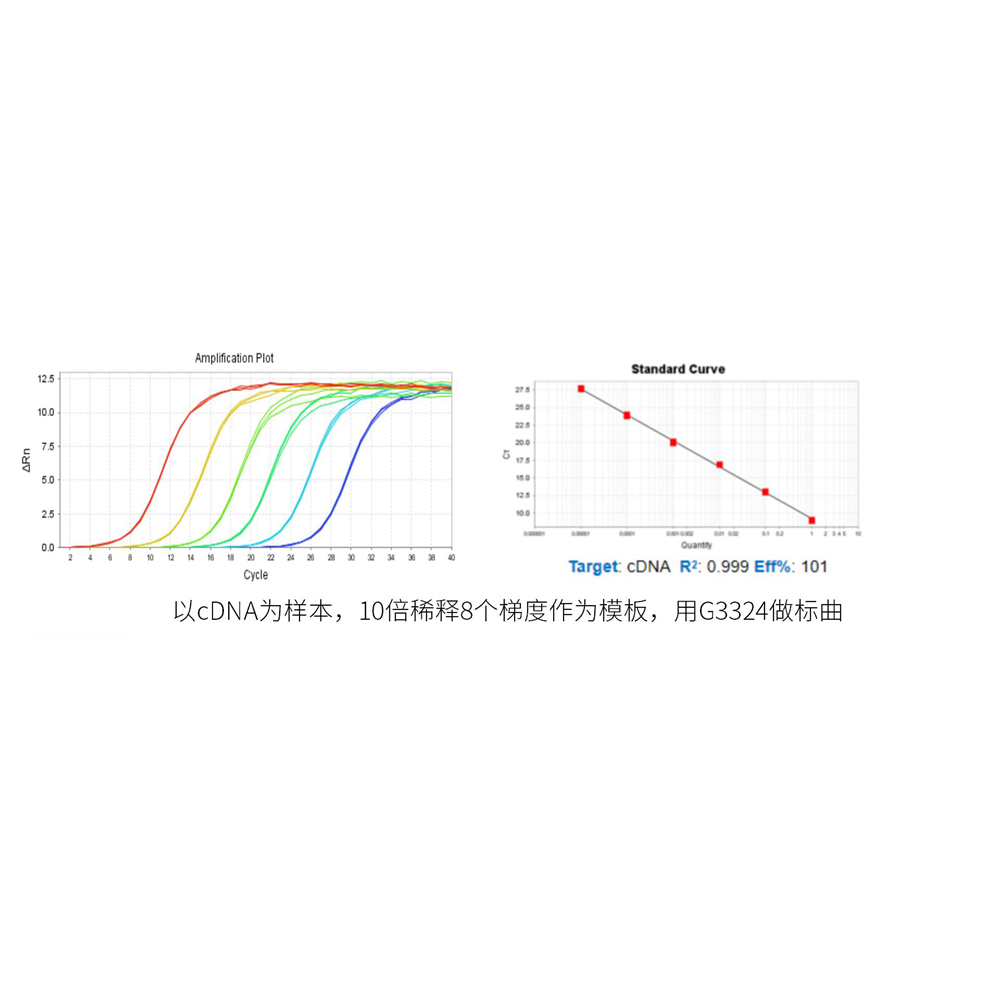
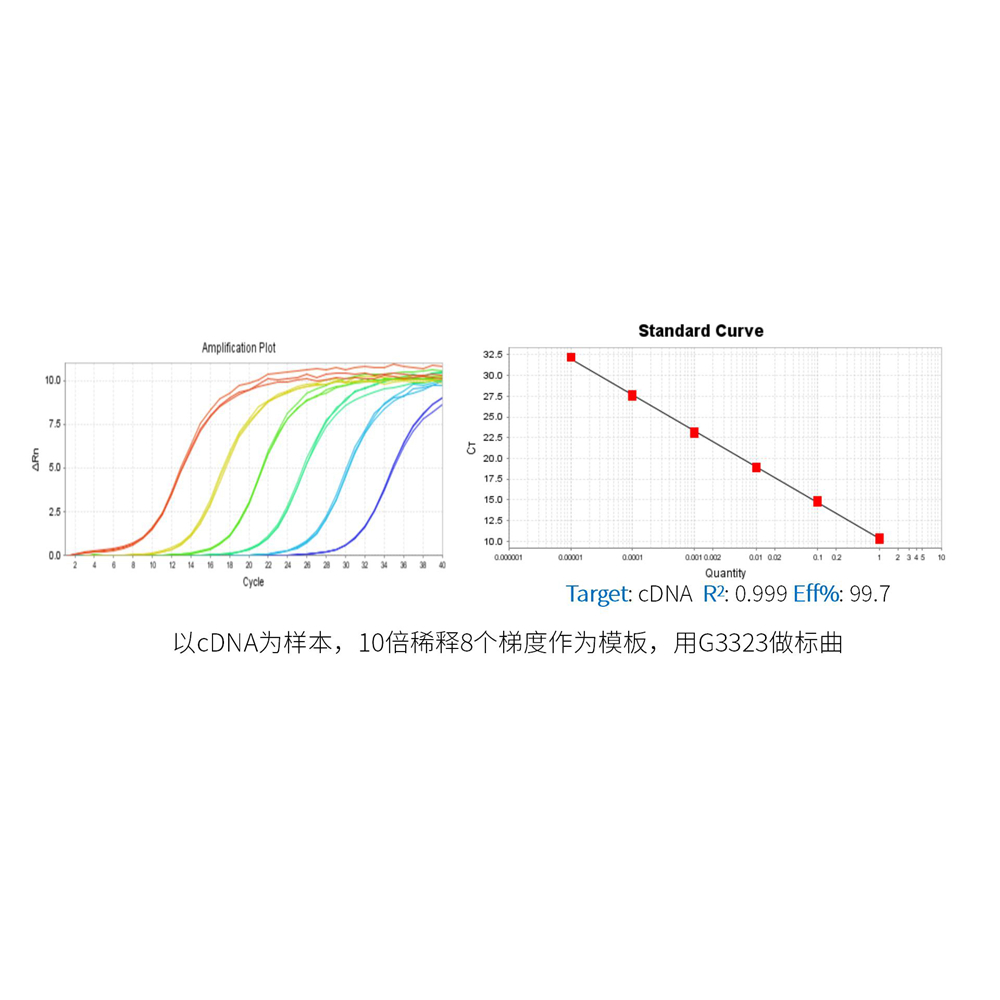
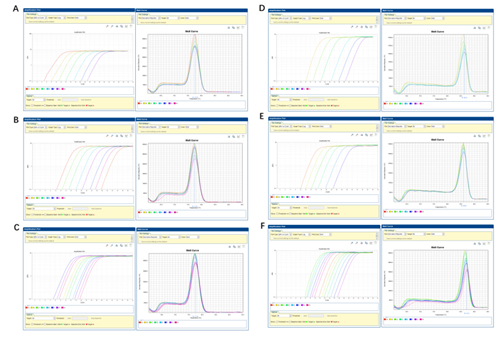
This product is a 2x premix solution for qPCR using SYBR Green I chimeric fluorescence method. The core component, Taq DNA Polymerase, is a heat-activated DNA Polymerase blocked by antibody method, which can effectively inhibit nonspecific amplification under low temperature conditions. Meanwhile, combined with the reaction Buffer optimized for qPCR, Taq DNA Polymerase is very suitable for high specificity and sensitivity qPCR reaction. A good standard curve can be obtained in a wide quantitative area, which is accurate, reproducible and reliable for the quantitative analysis of target genes. At the same time, this product contains a special ROX Passive Reference Dye which is suitable for use in all qPCR instruments, and there is no need to adjust the ROX concentration on different instruments. Blue dye is added in the premix, which has the tracer effect of adding samples, and yellow template diluent is provided at the same time. When the template is diluted with yellow template diluent and added into the blue reaction premix, the color changes from blue to green, which can play a tracer role in the preparation of reaction system process and prevent leakage or misaddition. The spectra of the blue and yellow dyes do not overlap with the qPCR dyes and do not affect the reaction results.
Storage and Handling Conditions
Wet ice bag transportation; Stored at -20℃ temperature, valid for 12 months.
Component
| Component Number | Component | G3326-01 | G3326-05 | G3326-15 |
| G3326-1 | 2x Universal Blue SYBR Green qPCR Master Mix | 1mL/5x1mL/15x1mL | 5x1mL | 15x1mL |
| G3326-2 | 40x Yellow Template Dilution Buffer | 1mL | 1mL | 1mL |
| Manual | 1pc | 1pc | 1pc | |
Assay Protocol / Procedures
1. (Optional) template dilution
This kit provides a 40x YELLOW Template Dilution Buffer, a 40-fold diluted Yellow Template Dilution that can be used to accurately determine whether the Template has been added to the qPCR reaction fluid by changing the color of the liquid. Taking the 20μL qPCR reaction system as an example, according to the dilution template amount added into the 20μL qPCR reaction solution, the corresponding dilution method of the original template can be referred to the following table (taking the original template diluted to 100μL as an example):
Example: 2μL diluted template should be added to 20μL qPCR reaction system. Taking the 100μL Template Dilution system as an example, when the original Template volume is 20μL, 25μL 40x Yellow Template Dilution Buffer is added, and then Nuclease-free Water 55μL is added to the total volume of 100μL. The 40x YELLOW TEMPLATE DILUTION BUFFER is now 10x. Add 2μL of the diluted Template into a 20μL Dilution Dilution Buffer, and the final 40× YELLOW Template Dilution Buffer is 1x. In conclusion, the Yellow Template Dilution Buffer should be 1x in the final qPCR system.
Note: If the template does not need to be diluted, or if the template diluent of G3362-2 is not used, you can ignore this step.
2. Recommend the qPCR reaction system:
a. Usually, a good amplification effect can be obtained with the final concentration of 0.2μM. When the reaction performance is poor, the primer concentration can be adjusted in the range of 0.2-1.0μM.
b. The amount of template addition varies with the copy number of the target gene in the template solution, and the appropriate amount of template addition is discussed by gradient dilution. The best addition amount of template DNA in the 20μL reaction system was less than 100 ng. When the cDNA (RT reaction solution) of RT-PCR reaction was used as template, the addition amount should not exceed 10% of the total volume of PCR reaction solution.
3. PCR reaction procedure (can be adjusted according to the instruments) :
a: If amplification specificity needs to be improved, two-step procedure or annealing temperature can be used; To improve the amplification efficiency, a three-step procedure or extension time can be used.
Note:
1. Please wear disposable gloves during operation to avoid RNase contamination.
2. The reverse transcription products can be stored at -20℃ for a short time. If long-term storage is needed, it is recommended to store them at -80℃ after packing to avoid repeated freeze-thaw cycles.
3. If the template is of eukaryotic origin, it is recommended to select Oligo (dT)18 Primer and pair it with the 3′ Poly A tail of eukaryotic mRNA to obtain the highest yield of full-length cDNA.
4. For reverse transcription of prokaryotic RNA, Random Hexamer Primer or Gene Specific Primer should be used.
5. Random Hexamer Primer has wide applicability and is suitable for mRNA, rRNA, tRNA, small RNA and lncRNA templates.
6. If reverse transcription is followed by qPCR assay, Oligo (dT)18 Primer and Random Hexamer Primer can be mixed to make cDNA synthesis efficiency in all regions of mRNA the same, which helps to improve the authenticity and repeatability of quantitative results.
7. If the subsequent qPCR primers are designed across exons, the genome removal step can be omitted.
Compatibility:
ABI: 5700, 7000, 7300, 7700, 7900, 7900HT, 7900 HT Fast, StepOne™, StepOne Plus™, 7500/7500 Fast, ViiA 7™, QuantStudio™ series, PikoRealTM Cycler;
Stratagene: Mx3000P®, 3005P™, 4000™;
Bio-Rad: CFX96™, CFX384™, iCycler iQ™, iQ5™, MyiQ™, MiniOpticon™, Opticon®, Opticon 2, Chromo4™;
Eppendorf: Realplex 2s, Mastercycler® ep, realplex;
IIIumina: Eco QPCR;
Cepheid: SmartCycler®;
Qiagen Corbett: Rotor-Gene® series;
Roche: LightCycler™ series;
Takara: Thermal Cycler Dice series;
Analytikjena: qTOWER series;
qTOWER: LineGene series.
Primer design principles:
1. The length of amplification product is recommended to be between 80-300 bp;
2. Primer length: 18-25 bp;
3. The content of base G+C in primers should be between 40%-60%;
4. The Tm value difference between forward primers and reverse primers is less than 2℃, and the Tm value between 58-62℃ is the best;
5. Randomness of base distribution;
6. Primers had better not contain self-complementary sequences, otherwise they will form a secondary hairpin structure;
7. There should be no more than 4 complementary or homologous bases between two primers, otherwise primer dimer will be formed, especially complementary overlap at the 3′ end;
8. The 3′ terminal base of the primer is suggested to be G or C;
9. No other non-specific products were found in NCBI comparison results.
Common problems and solutions:
| Problem description | Possible reasons | Solutions |
| At the end of the reaction, no amplification curve appeared or CT value appeared too late | The template concentration is too low | Repeat the experiment to reduce the template dilution multiple, and start from the highest concentration when the sample concentration is unknown |
| Template degradation | The template was prepared again and the experiment was repeated | |
| There are PCR inhibitors in the system | Generally, the template is carried in, the dilution ratio of the template is increased or the template with high purity is reprepared and repeated | |
| Primers may degrade | Primers that have not been used for a long time should first be tested for integrity by PAGE electrophoresis to rule out the possibility of degradation | |
| Low amplification efficiency | ncrease the primer concentration, try a three-step amplification procedure, or redesign the primer | |
| The amplification product is too long | The amplification product length was controlled in the range of 80-300 bp | |
| The blank control shows the signal | Reaction system pollution | Firstly, the blank control water should be replaced. If the same situation still occurs, the primers, aspirators and PCR tubes should be replaced or a new Master Mix should be started. The reaction system is prepared in a super clean table to reduce aerosol pollution |
| Non-specific amplification such as primer dimers appears | Generally, it is normal for the amplification products to appear in blank control after 35 cycles, which should be analyzed with the melting curve.
Redesign primer, adjust primer concentration or optimize PCR reaction procedure |
|
| The melting curve has multiple peaks | Primer design is poor | The new primer was re-designed according to the primer design principles |
| Primer concentration is too high | Reduce primer concentration appropriately | |
| There is genomic contamination in cDNA template | The extracted RNA solution is digested using DNA enzymes, such as DSDNase, to remove genomic contamination, or to design transintron primers | |
| Poor reproducibility of experiments | The error of adding sample is large | The use of accurate pipette, with high quality suction head accurate pipette;
High dilution template, adding large volume template to reduce sampling error; The reaction volume of qPCR was enlarged |
| The template concentration is too low | Repeat the experiment to reduce the dilution times of the template | |
| Temperature deviation at different locations of the qPCR instrument | Calibrate the qPCR instrument regularly | |
| The amplification curve is not smooth | Fluorescence signal is too weak, produced after system correction | Ensure that the dyes premixed in the Master Mix are not degraded;
Replace fluorescent signal to collect better qPCR consumables |
| Amplification curve breaks or slips | The template concentration was higher and the baseline endpoint value was greater than the CT value | The baseline endpoint (Ct value -3) was reduced and the data were reanalyzed |
| Amplification curves of individual Wells suddenly dropped sharply | There are bubbles in the reaction tube | Ensure that MIX is completely dissolved, and do not swirl and oscillate evenly;
After the sample is added, the bubbles are removed by centrifugation with light elastic. The pre-denaturation time was extended to 10 min to remove the bubbles |
For Research Use Only!
Ver. No.: V1.0-202111
|
Cat.No.
|
Product Name
|
Spec.
|
Operation
|
|---|
|
G3326-01
|
2×Universal Blue SYBR Green qPCR Master Mix With 40×Yellow Template Dilution Buffer
|
1 mL
|
|
|
G3326-05
|
2×Universal Blue SYBR Green qPCR Master Mix With 40×Yellow Template Dilution Buffer
|
5×1 mL
|
|
|
G3326-15
|
2×Universal Blue SYBR Green qPCR Master Mix With 40×Yellow Template Dilution Buffer
|
15×1 mL
|
|
|
G3327-01
|
2×Universal Blue Probe qPCR Master Mix
|
1 mL
|
|
|
G3327-05
|
2×Universal Blue Probe qPCR Master Mix
|
5×1 mL
|
|
|
G3327-15
|
2×Universal Blue Probe qPCR Master Mix
|
15×1 mL
|
|
|
G3328-01
|
2×Universal Blue SYBR Green qPCR Master Mix (with UDG)
|
1 mL
|
|
|
G3328-05
|
2×Universal Blue SYBR Green qPCR Master Mix (with UDG)
|
5×1 mL
|
|
|
G3328-15
|
2×Universal Blue SYBR Green qPCR Master Mix (with UDG)
|
15×1 mL
|