Description
linearized polyethy leneimine
Description
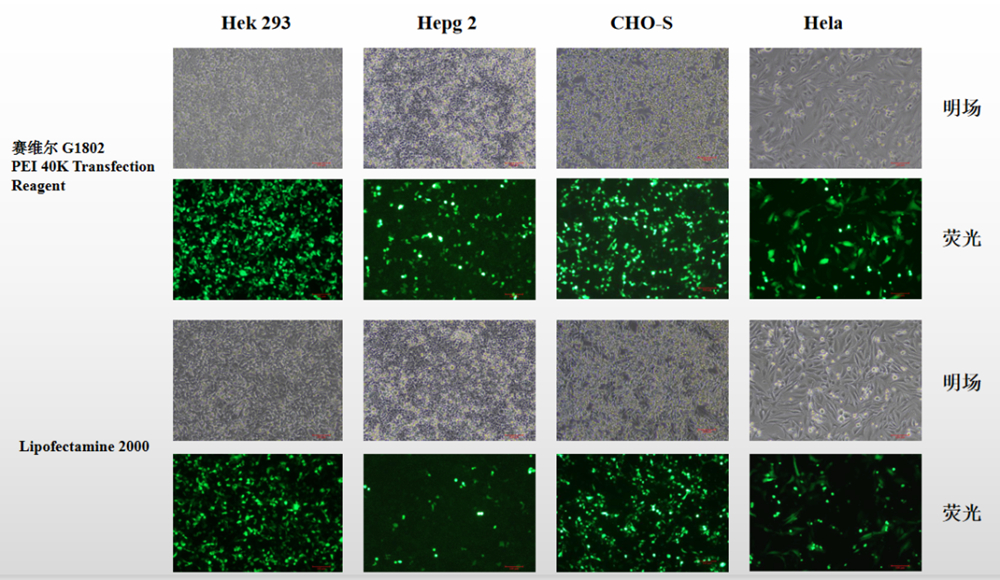
Transfection of DNA into eukaryotic cells is a common method to study biological mechanisms. A major goal is the efficient and specific delivery of genes into the desired target cells. Although a wide panel of techniques and vectors (viral and nonviral) have been developed that work with variable efficiency, most vectors lack a target cell specificity. Nonviral vectors are attractive because of their ease of manipulation, safety, and high flexibility in the size of the delivered transgene. The Transferrinfection is a high-efficiency nucleic acid delivery system based on transferrin receptor-mediated endocytosis to carry DNA into cells. Furthermore it was shown that the cationic polymer polyethylenimine (PEI) mediates efficient gene transfer into a variety of cells. In the Polyethylenimine-Transferrinfection system, the gene transfer efficiency of PEI/DNA complexes are combined with the specific mechanism of receptor-mediated endocytosis via Transferrin receptor.
The method results in a 30–1,000-fold enhanced transfection efficiency depending on the cell line. It is an extremely gentle DNA transfection method that employs physiological uptake mechanisms of the cell. Transfection efficacy depends on the cell type, the level of surface transferrin receptor expression. Very high transfection rates have been shown for the tested tumor cell lines B16F10 melanoma, Neuro 2A neuroblastoma, and a variety of primary human melanoma cell lines. In other established cell lines, such as HeLa, CHO, Jurkat, K562, HepG2, and COS, the PEI-Transferrinfection works with high efficiences, excellent reproducibility and with the advantage of being an extremely gentle procedure.
Human transferrin is covalently linked to a polycationic carrier (Polyethylenimine/PEI) with intrinsic/ inherent endosomal activity. PEI has the capacity to condense DNA and deliver it into cells, presumably by adsorptive endocytosis. Furthermore, PEI is only partially protonated at physiological pH. Upon acidification within the endosome/ lysosome, PEI presumably acts as proton sponge with the protonation triggering chloride influx, osmotic swelling, and destabilization of the endosomal/ lysosomal vesicle enabling the release of the DNA into the cytosol. As a result, efficient gene transfer is obtained without the need for additional endosome destabilizing agents. The modified, PEI-conjugated, transferrin molecules maintain their ability to bind their cognate receptor and to mediate efficient iron transport into the cell. Transferrin receptor-mediated endocytosis occurs followed by the expression of the imported DNA. Deferrioxamine, a cell permeable iron chelator, increases the transferrin receptor density on the cell surface thus further enhancing gene delivery to the cell.
The concentration of Pei 40K, the main component of this product, is 1 mg / ml.
Storage and Handling Conditions
Wet ice bag transportation; Stored at -20 ℃, valid for 6 months; Alternatively, Stored at 4 ℃,valid for 3 months.
| Component Number | Component | G1802-1ML | G1802-10ML |
| G1802-1 | PEI 40 K Transfection Reagent | 1 mL | 10 mL |
| Instruction Manual | 1 pc | 1 pc | |
Assay Protocol / Procedures
1. Preparation before transfection of adherent cells (6-well plate, reference table for other specifications):
A) The day before transfection, cells were appropriate to be uniformly planted in the pore plates at an
appropriate density to converge to 70 – 85% upon formal transfection;
B)Before transfection, primary cell media was removed and washed 1-twice with PBS before replacing 1.8 mL of base medium without serum or low serum (below 5%), or Opti-MEM; different cells had different serum dependence depending on the specific cell type;
C)The apped cells were placed in the incubator and preparation of transfection complexes was started.
2. Suspension cells were prepared before transfection:
A) On the day of transfection, an appropriate amount of cells were centrifuged to remove the medium
and resuspended with basal medium without serum or low serum (below 5%);
B) The prepared suspension cells were placed in the incubator and started the preparation of
transfection complexes, and the volume ratio between transfection complexes and medium could
be made with reference to adherent cells for appropriate adjustments.
3. Transfection complexes were prepared:
A) Preparation liquid A: 100 μL of serum-free base medium and 2 μg of plasmid DNA, blowing and
mixed;
B)Preparation of liquid B: 100 L of base medium without serum and 6-8 μL PEI 40 K Transfection
Reagent, blowing and mixing;
C)Solution B was added to liquid A, gently blown and mixed, and incubated at room temperature for
15 min to form a DNA-PEI transfection complex.
4. Transfected cells:
A) Add the transfection complex obtained in the previous step to the previously changed hole plate by
crossing or surrounding the solution;
B) Shake the plate “8” or other way to be fully mixed and incubated in a 37℃, 5% CO2 incubator;
C) DNA-PEI transfection complexes with cells for 4 – 6 h showed better transfection efficiency,
appropriate extension or overnight incubation and better transfection efficiency.
Note
1. Please use healthy cells in good condition and recovered cells are recommended to transage at least 2 times before transfection.
2. Please use a high-quality, endotoxin-free, plasmid DNA.
3. High concentrations of serum at transfection, as well as the use of antibiotics, may affect cell status as well as transfection efficiency.
4. Different cell tolerance, sensitivity, length of transfection incubation time, and PEI / DNA usage ratio were adjusted as appropriate.
5. Please wear experimental suits and disposable gloves when operation.
Schedule: Use of transfection in different cell culture containers (for reference only)
| Cultivate utensils | Growth area
(cm2) |
Number of inoculated cells | DNA content(μg) | PEI
(μL) |
Transfection complex volume
(μL) |
Total volume(mL) |
| 96-well plates | 0.3 | (2-4)×104 | 0.1 | 0.3-0.4 | 10 | 0.1 |
| 24-well plates | 1.9 | (1.2-2.4)×105 | 0.5-1 | 1.5-4 | 50 | 0.5 |
| 12-well plates | 3.8 | (2.4-4.8)×105 | 1-2 | 3-8 | 100 | 1 |
| 6-well plates | 9.5 | (6-10)×105 | 2-4 | 6-16 | 200 | 2 |
| 10 cm culture dish | 55 | (4-6)×106 | 12-24 | 36-96 | 1000 | 12 |
| T75 culture flask | 75 | (6-10)×106 | 18-36 | 54-144 | 1000 | 15 |
For Research Use Only!