Description
Product Information
Product Name: FITC-labeled Phalloidin (Green Fluorescence) Product Number: G1028-100T Size: 100 tests
Product Description
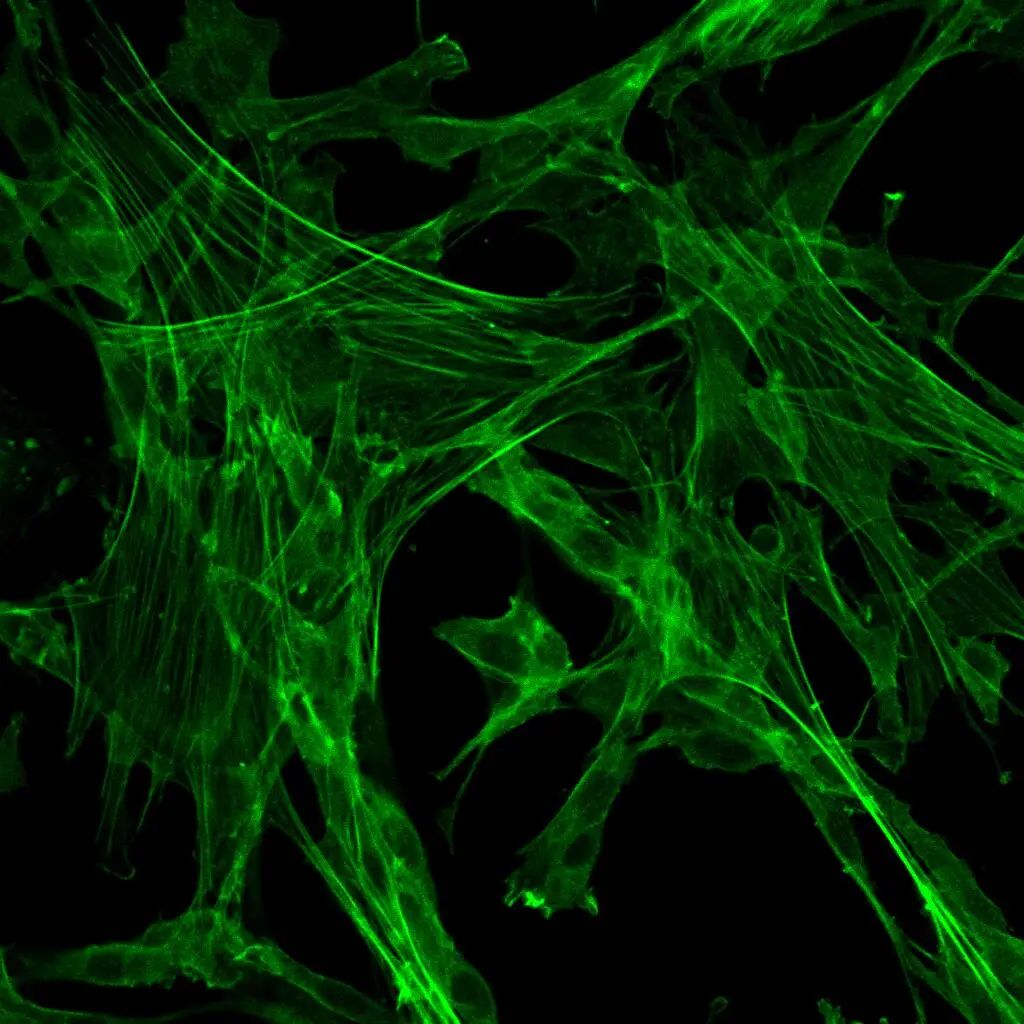
Phalloidin, also known as Phalloidin toxin, is a bicyclic peptide initially isolated from the toxic mushroom Amanita phalloides. It exhibits a high affinity and specificity for binding to F-actin, a component of muscle fibers, with no binding to G-actin. Phalloidin demonstrates similar affinities for different fiber sizes, and its binding ratio typically involves one phalloidin molecule per actin subunit. Phalloidin has minimal non-specific binding, resulting in distinct staining of both stained and unstained regions. It can stain F-actin at nanomolar concentrations, making it useful for labeling, identifying, and quantifying F-actin distribution.
This product is FITC-labeled Phalloidin provided in a 20 μM storage solution format with DMSO as the solvent. The recommended working concentration range is 80-200 nM, using 200 μL of working solution per staining, resulting in a 100 nM working concentration. This product allows for 100 cell staining procedures.
Storage and Transportation
Shipped with ice packs (wet ice) and stored at -20°C in the dark. Shelf life is 12 months.
Components
- G1028-100T FITC-labeled Phalloidin (Green Fluorescence) 100 tests
- Instruction Manual 1 copy
Preparation Before Experiment
Prepare 1× PBS buffer (pH 7.2-7.4, recommended G4202), BSA (recommended GC305010), fixative solution (containing 4% paraformaldehyde, recommended G1101), permeabilization solution (0.1%-0.5% Triton X-100 in PBS, recommended G1204), anti-fade mounting medium (recommended G1401), and ready-to-use DAPI staining solution (recommended G1012), etc.
For the first use of this product, thaw the product thoroughly and centrifuge at low speed for 1 minute to prevent liquid loss along the tube wall. It is recommended to aliquot based on the amount needed for a single experiment to prevent solvent evaporation loss. Store at -20°C in the dark.
Before the formal experiment, prepare 1% BSA-containing PBS by dissolving 1.0 g of BSA in 100 mL of PBS. Mix 1 μL of FITC-labeled Phalloidin with 199 μL of 1% BSA-containing PBS to obtain a 100 nM working solution. The commonly used working concentration is 80-200 nM, with 100 nM recommended. The optimal staining concentration can be determined through literature references or preliminary experiments. The working solution of FITC-labeled Phalloidin can be used on the same day, stored at room temperature in the dark.
Experimental Procedure
- Plate cultured cells (density at least half-confluent), remove the culture medium, and wash cells twice with pre-warmed 1× PBS buffer at 37°C.
- Cover cells with fixative solution containing 4% paraformaldehyde at room temperature and fix for 10-30 minutes. Note that methanol can disrupt actin, so the fixative solution should not contain methanol.
- Wash cells with 1× PBS buffer at room temperature for 2-3 times, 10 minutes each time.
- Cover cells with permeabilization solution at room temperature and permeabilize cells for 5 minutes.
- Wash cells with 1× PBS buffer at room temperature for 2-3 times, 10 minutes each time.
- Gently shake the cell plate to position cells in the center of the circle. Cover cells completely with 200 μL of the freshly prepared FITC-labeled Phalloidin working solution and incubate at room temperature in the dark for 30 minutes. To prevent solution evaporation and maintain humidity during incubation, place the cell plate in a sealed container, such as a light-protected humidified chamber. Additionally, adding 1% BSA to the Phalloidin staining solution can effectively reduce background staining.
- Wash cells with 1× PBS buffer for 2-3 times, 5 minutes each time.
- (Optional) Cover cells with ready-to-use DAPI staining solution for 3-5 minutes to stain cell nuclei. Wash cells with 1× PBS buffer for 2-3 times, 30 seconds to 1 minute each time.
- Gently dry the cell plate, invert it onto a glass slide with a drop of anti-fade mounting medium, and remove excess mounting medium with a tissue.
[Optional step]: After completing step 7, you can also directly place the cover glass slide with DAPI-containing anti-fade mounting medium (recommended G1407) onto the cell plate, then remove excess mounting medium. Observe the results using a fluorescence microscope or confocal laser scanning microscope.
- Observe the results using a fluorescence microscope or confocal laser scanning microscope, and select FITC (Ex/Em=492/518 nm) and DAPI (Ex/Em=364/454 nm) channels.
Notes
- Fluorescent dyes can undergo quenching, so it is advisable to complete detection and observation soon after staining.
- Methanol can disrupt actin, so fixative solutions containing methanol should not be used during initial fixation.
- Phalloidin generally lacks cell permeability and is rarely used for live cell staining.
- The solvent of this product is methanol, which evaporates easily. Seal the cap tightly after each use to prevent evaporation.
- Wear laboratory attire and disposable gloves when performing the procedure.
This product is for research purposes only and not intended for clinical diagnosis.