Description
Product Information
| Product Name | Cat.No. | Spec. |
| 2×In-Fusion Cloning Mix | G3350-20T | 20 T |
| G3350-100T | 100 T |
Description
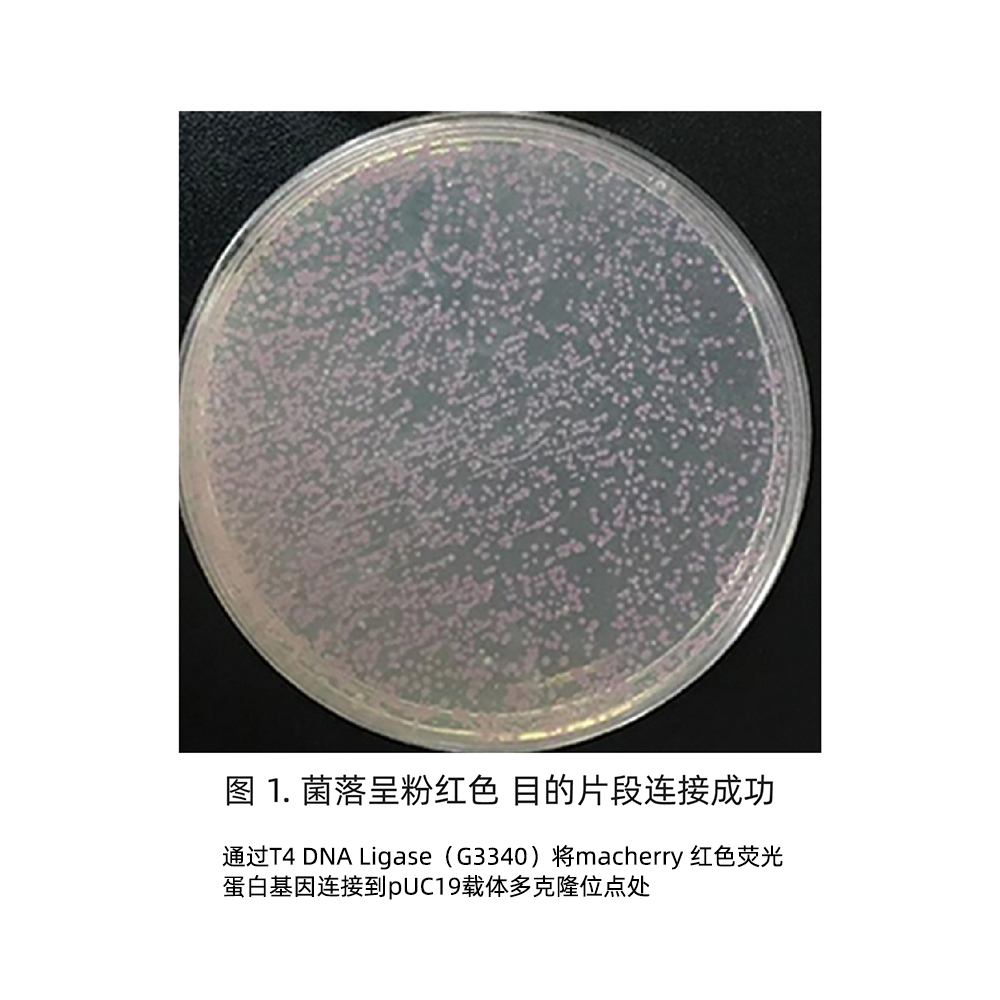
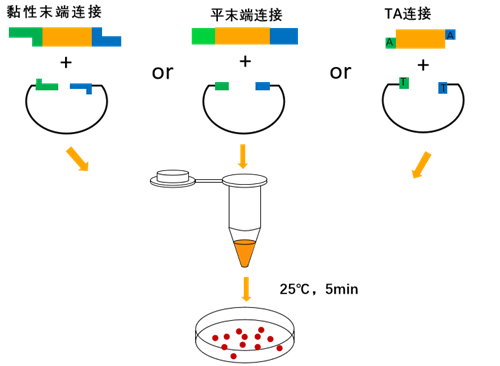
2×In-Fusion Cloning Mix can rapidly ligate single or 2~3 multiple fragments into the vector and clone into the vector, especially suitable for single fragment ligation, and the efficiency of 2-3 fragment ligation will be reduced. Its principle is to linearize the vector first, and then introduce the end sequence of the linearized vector into the 5′ end of the forward/reverse amplification primer of the DNA fragment inserted into the linearized vector, so that the 5′ and 3′ ends of the PCR amplification product are Each has a sequence (15-25 bp) identical to the end of the linearized vector. In the 2×In-Fusion Cloning Mix master mix, it is necessary to mix the PCR product and the linearized vector with sequences consistent with the ends of the linearized vector in a certain proportion, and then incubate it on ice (or in ice water mixture) for 5 min. Then, transformation of competent cells can be performed and directed cloning can be accomplished.
This premix can realize efficient and rapid DNA seamless cloning, and it can be used for the construction of recombinant plasmids or gene point mutation recombinant plasmids. This premix does not depend on the ligase system, which greatly reduces the background of vector self-ligation, and it does not need to consider the restriction endonuclease site contained in the inserted fragment itself. For the construction of single-segment DNA recombinant plasmids, the proportion of positive clones obtained using this master mix is as high as 99%. When using this premix solution, it does not depend on constant temperature equipment, and can complete all operations from DNA sample preparation to transformation plating culture of recombinant products within a few hours, which greatly saves time.
Storage and Handling Conditions
Transported with wet ice; stored at -20°C, valid for 12 months.
Component
| Component | G3350-10T | G3350-20T | G3350-100T |
| 2×In-Fusion Cloning Mix | 50 μL | 100 μL | 5×100 μL |
| pUC19 (Linearized, Control Vector, 5 ng/μL) | – | 10 μL | 10 μL |
| Control Insert (10 ng/μL) | – | 10 μL | 10 μL |
| Product Manual | |||
Assay Protocol
Ligation system (recommended 10 μL reaction system)
| Component | Volume |
| 2×In-Fusion Cloning Mix | 5 μL |
| Vector | X μL |
| DNA Segment | Y μL |
| Nuclease-Free Water | Add To 10 μL |
Reaction conditions
Ice-water bath (ice-water mixture), react for 5-10 min.
Reaction product conversion
1. Take out the competent cells (such as E.coli DH5α, E.coli Top10, etc.) from the -80℃ refrigerator and place them on ice to thaw.
2. Add the reacted sample to the competent state, gently move the bottom of the tube with your fingers to mix well, and take an ice bath for 30 minutes.
3. The product was then placed in a water bath at 42°C and heat-shocked for 90 s. After that, it was quickly placed on ice for 2-5 minutes.
4. Take 900 μL of sterile SOC or LB medium and add it to the EP tube. After mixing, place the EP tube on a shaker and incubate at 220 rpm at 37°C for 1 h to recover the bacteria (it can also be cultured in a 37°C incubator for 1 h);
5. According to the experimental requirements, draw different volumes of transformed competent cells and add them to LB solid medium containing corresponding antibiotics, spread the cells evenly, and after the liquid is completely absorbed, place the plate upside down in a 37°C incubator and culture overnight.
Positive clone identification
The monoclonal colonies grown on the plates are picked for colony PCR identification, or after culturing, the plasmids are extracted for digestion or PCR identification, or the extracted plasmids are directly sequenced and identified.
Note:
1. The vector and target fragments must be gel purified and their quality and concentration detected by electrophoresis. Water can be omitted when the concentration is low.
2. The Tm value between the overlapping regions of multiple fragments should be consistent and >60℃.
3. It is recommended that the molar ratio of vector and insert is 1:1~1:3; when 2-3 fragments are connected, the molar ratio between each fragment is 1:1, and the ligation efficiency of 2-3 fragments will decrease. The ligation reaction system can be scaled up in equal proportions. .
4. When the total volume of vector and insert is greater than 5 μL, the reaction system can be scaled up to 20 μL.
5. The volume of the ligation product should not exceed 1/10 of the volume of the competent cells, otherwise the transformation efficiency will be significantly reduced. The volume of the ligation product and the competent cells can be increased in equal proportions (for example, 20 μL of ligation system transforms 200 μL of competent cells).
6. It is recommended to take out the 2×In-Fusion Cloning Mix before use, and put it back to -20°C immediately after use. It can be frozen in aliquots to reduce repeated freeze-thaw cycles.
7. When electroporation is used for transformation, the reaction product needs to be purified by column method or ethanol precipitation method.
For Research Use Only!
Ver. No.: V1.0-202111
Assay Protocol
Ligation system (recommended 10 μL reaction system)
| Component | Volume |
| 2×In-Fusion Cloning Mix | 5 μL |
| Vector | X μL |
| DNA Segment | Y μL |
| Nuclease-Free Water | Add To 10 μL |
Reaction conditions
Ice-water bath (ice-water mixture), react for 5-10 min.
Reaction product conversion
1. Take out the competent cells (such as E.coli DH5α, E.coli Top10, etc.) from the -80℃ refrigerator and place them on ice to thaw.
2. Add the reacted sample to the competent state, gently move the bottom of the tube with your fingers to mix well, and take an ice bath for 30 minutes.
3. The product was then placed in a water bath at 42°C and heat-shocked for 90 s. After that, it was quickly placed on ice for 2-5 minutes.
4. Take 900 μL of sterile SOC or LB medium and add it to the EP tube. After mixing, place the EP tube on a shaker and incubate at 220 rpm at 37°C for 1 h to recover the bacteria (it can also be cultured in a 37°C incubator for 1 h);
5. According to the experimental requirements, draw different volumes of transformed competent cells and add them to LB solid medium containing corresponding antibiotics, spread the cells evenly, and after the liquid is completely absorbed, place the plate upside down in a 37°C incubator and culture overnight.
Positive clone identification
The monoclonal colonies grown on the plates are picked for colony PCR identification, or after culturing, the plasmids are extracted for digestion or PCR identification, or the extracted plasmids are directly sequenced and identified.
Note:
1. The vector and target fragments must be gel purified and their quality and concentration detected by electrophoresis. Water can be omitted when the concentration is low.
2. The Tm value between the overlapping regions of multiple fragments should be consistent and >60℃.
3. It is recommended that the molar ratio of vector and insert is 1:1~1:3; when 2-3 fragments are connected, the molar ratio between each fragment is 1:1, and the ligation efficiency of 2-3 fragments will decrease. The ligation reaction system can be scaled up in equal proportions. .
4. When the total volume of vector and insert is greater than 5 μL, the reaction system can be scaled up to 20 μL.
5. The volume of the ligation product should not exceed 1/10 of the volume of the competent cells, otherwise the transformation efficiency will be significantly reduced. The volume of the ligation product and the competent cells can be increased in equal proportions (for example, 20 μL of ligation system transforms 200 μL of competent cells).
6. It is recommended to take out the 2×In-Fusion Cloning Mix before use, and put it back to -20°C immediately after use. It can be frozen in aliquots to reduce repeated freeze-thaw cycles.
7. When electroporation is used for transformation, the reaction product needs to be purified by column method or ethanol precipitation method.
For Research Use Only!
Ver. No.: V1.0-202111
|
Cat.No.
|
Product Name
|
Spec.
|
Operation
|
|---|
|
G3022-25T
|
pSWE-Topo Zero Cloning Kit
|
25 T
|
|
|
G3340-100
|
T4 DNA Ligase (5 U/μL )
|
500 U
|
|
|
G3340-50
|
T4 DNA Ligase (5 U/μL )
|
250 U
|
|
|
G3341-100
|
2 x Universal Ligation Mix
|
100 T
|
|
|
G3341-50
|
2 x Universal Ligation Mix
|
50 T
|
|
|
G3350-100T
|
2×In-Fusion Cloning Mix
|
100 T
|
|
|
G3350-20T
|
2×In-Fusion Cloning Mix
|
20 T
|
|
|
G3351-100T
|
2×In-Fusion Cloning Mix Plus
|
100 T
|
|
|
G3351-20T
|
2×In-Fusion Cloning Mix Plus
|
20 T
|
|
|
G3400-1000U
|
Alkaline Phosphatase (Thermosensitive)
|
1000 U
|