Description
Product Information
| Product Name | Cat.No | Spec. |
| Mycoplasma Elimination Reagent (SweMyco-1+2) (100×) | G4008-5ML | 5 mL |
Introduction
This product is a combination of quinolones, tetracyclines and macrolides. It can effectively eliminate mycoplasma by inhibiting DNA replication and protein synthesis of mycoplasma. Through optimization and mode, this product not only has low cytotoxicity and fast mycoplasma clearance rate, but also can completely remove mycoplasma contained in cultured cells in about 7 days. After changing the normal medium, the cells are in good condition, and there is no repulsion phenomenon in continuous multi-generation culture.
Storage and Handling Conditions
Transport with wet ice; Store at -20℃, avoid repeated freezing and thawing, valid for 12 months.
Components
| Component No. | Component | G4008-5ML |
| G4008-1 | SweMyco Elimination Reagent-1 (100×) | 5×1 mL |
| G4008-2 | SweMyco Elimination Reagent-2 (100×) | 5×1 mL |
| Instruction Manual | 1 pc | |
Usage
1. Preparation of medium A: Add 1 mL SweMyco Elimination Reagent-1 (100×) to 100 mL cell media (containing serum, without double antibody), and mix thoroughly;
2. Preparation of medium B: add 1 mL SweMyco Elimination Reagent 2 (100×) to 100 mL cell media (containing serum, without double antibody), and mix thoroughly;
3. When the mycoplasma infected cells were subcultured or the liquid was changed, culture medium A was used for routine cell culture for 4 days; Then the cells were conventionally cultured for 3 days using medium B;
4. After one round of culture (7 days), some cells are seeded into a 6-well plate (or other culture vessel) and replaced with complete medium without SweMyco Elimination Reagent. The remaining cells were continued to use medium A and B alternately for cell culture;
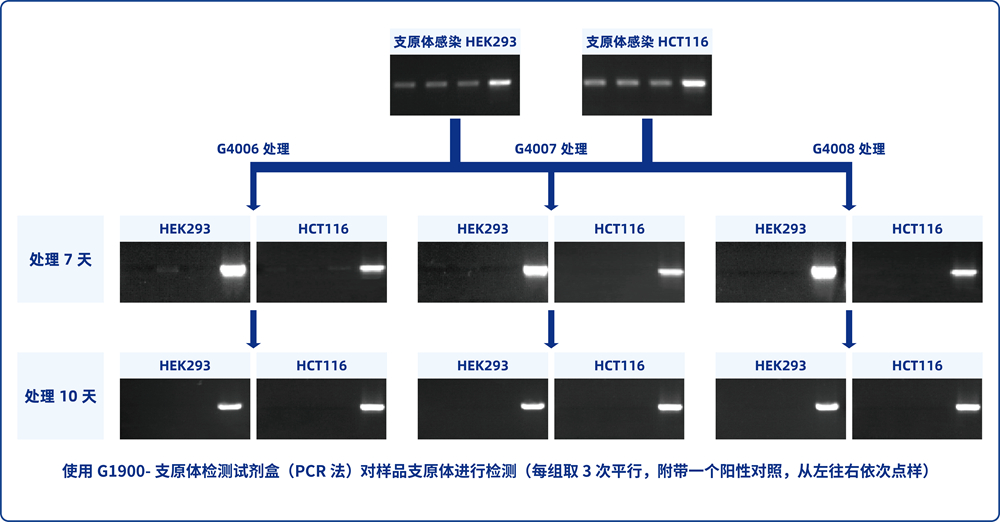
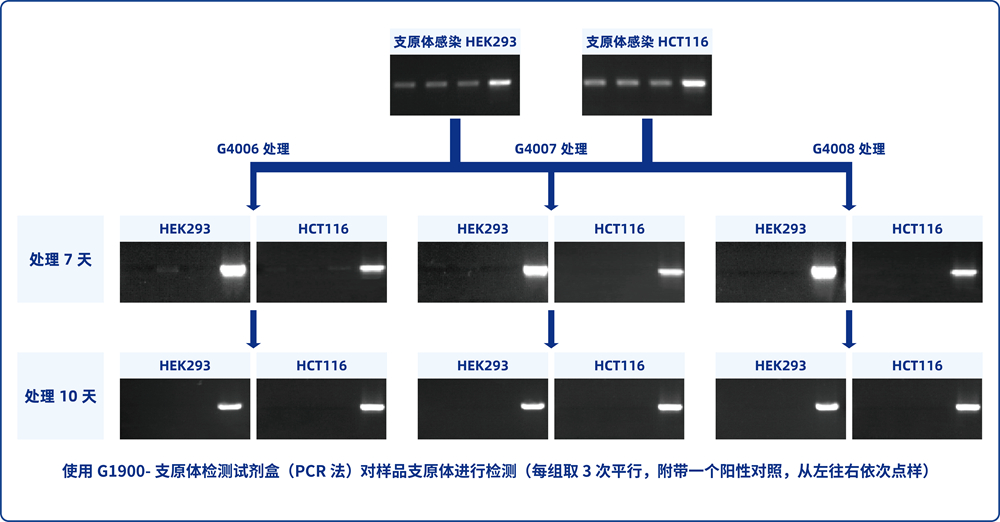
5. Cells in 6-well plates were cultured for 2-3 days without SweMyco Elimination Reagent medium, and mycoplasma detection was performed when the confluent degree of cells reached more than 90% (G1900, G1901, and G1902 are recommended).
6. According to the test result, perform the following operations:
A) Positive result: continue to repeat steps 3-5 until the test result is negative.
B) Negative result: the cell group containing SweMyco Elimination Reagent medium is replaced with normal complete medium, and mycoplasma detection is performed after culture for a period of time. Negative retest result indicates completion of Elimination.
Note
1. This product has been tested in a variety of mycoplasma infected cell lines, but different cell lines have different sensitivity to reagents. If the cytotoxicity is too great or the removal effect is not good, the solubility of the product can be adjusted appropriately.
2. There are many ways for cells to be infected with Mycoplasma. It is recommended to add mycoplasma preventive reagent (G4009 is recommended) in daily culture.
3. For cells that have been identified as mycoplasma contamination, separate ultra-clean tables should be used as far as possible to avoid cross-contamination.
4. For your safety and health, please wear a lab coat and disposable gloves when operating.
Attached Table: Features of Mycoplasma Removal Series
| Product Name | Mycoplasma Elimination Reagent (100×) | Mycoplasma Elimination Reagent Plus (100×) | Mycoplasma Elimination Reagent (SweMyco-1+2) (100×) |
| Product Code | G4006 | G4007 | G4008 |
| Composition | Quinolones、Tetracycline | Quinolones, Tetracyclines, Macrolides | Quinolones, Tetracyclines, Macrolides |
| Relative toxicity | Middle | High | Low |
| Clear speed | About 10 days | 5-7 days | About 7 days |
| Ease of use | Easy | Easy | More complicated |