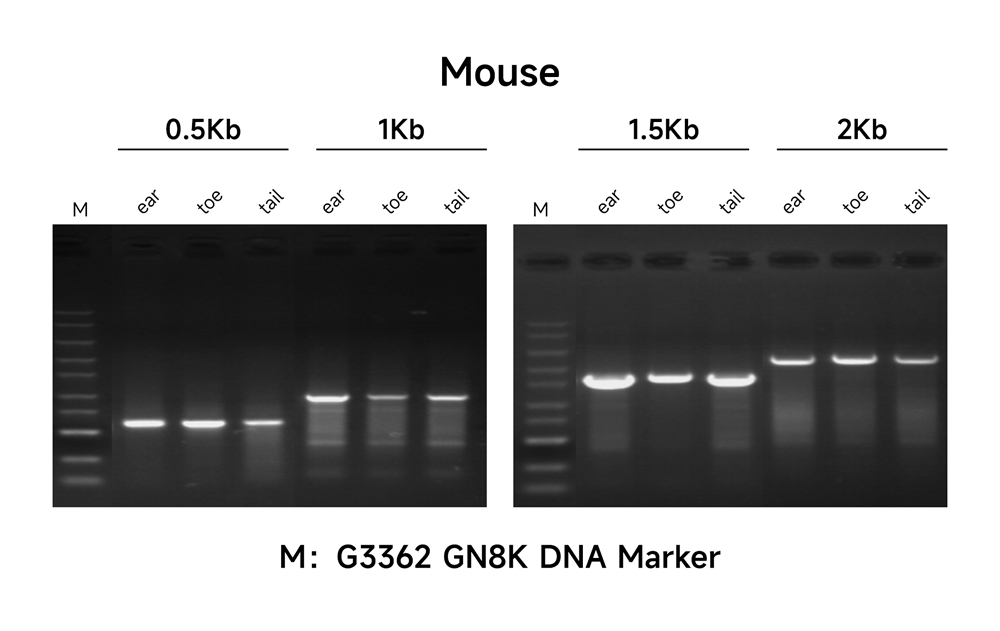
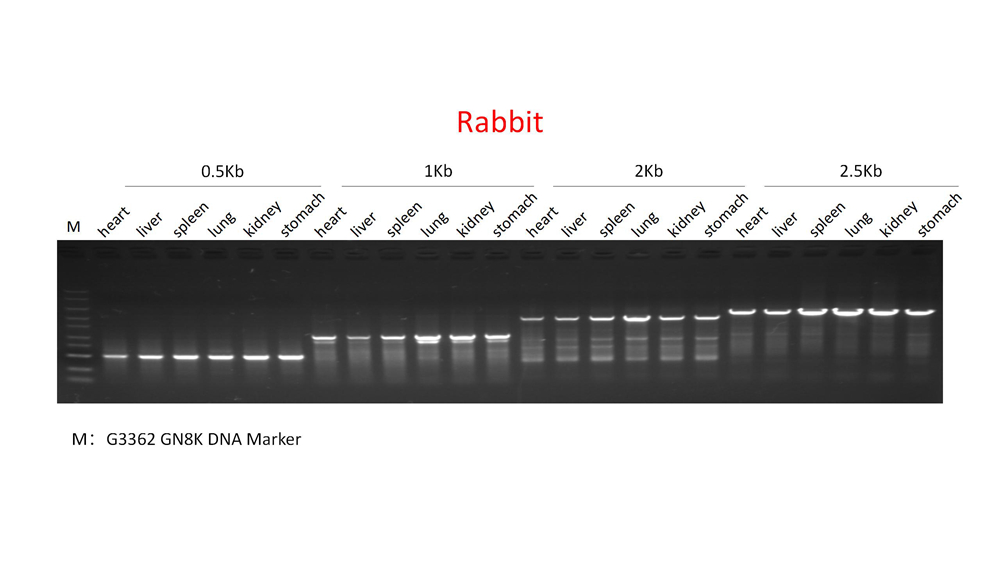
Description
Product Information
| Product Name | Cat.No. | Spec. |
| 2 x LA PCR Master Mix | G3307-01 | 1 mL |
| G3307-05 | 5×1 mL |
Description/Introduction
This product is a 2× premix solution containing LA DNA polymerase, reaction buffer and dNTPs, which facilitates the rapid preparation of PCR reaction system. LA DNA Polymerase is a hybrid enzyme consisting of a modified Taq DNA Polymerase with a DNA Polymerase containing a 3’→5′ exonuclease activity, which has 6 times the fidelity of the Taq DNA Polymerase and an amplification rate of approximately 10 s/kb. The combination of rapid amplification and high continuous synthesis capability with specially optimized reaction buffer can amplify target DNA fragments up to 30 KB from the genome, and have high amplification efficiency for different source and different length templates. For target fragments less than 10kb, the yield is significantly increased. The 3 ‘end of the amplified product contained “A” base and could be directly cloned into the T-vector. This product contains two tracer dyes and can be directly selected for agarose gel electrophoresis after PCR amplification.
Storage and Handling Conditions
Transport with wet ice. Store at -20°C, valid for 12 months.
Component
| Component | G3307–01 | G3307–05 |
| 2× LA PCR Master Mix | 1 mL | 5×1 mL |
| Manual | 1pc | |
Assay Protocol / Procedures
1.Recommended PCR reaction system:
| Component | 20 μL | 50 μL | Final Concentration |
| Templatea | Variable | Variable | as required |
| Forward Primer (10 μM)b | 1.0 μL | 2.5 μL | 0.5 μM |
| Reverse Primer (10 μM)b | 1.0 μL | 2.5 μL | 0.5 μM |
| 2×LA PCR Master Mix | 10 μL | 25 μL | 1× |
| (DMSO, optionalc) | (0.6 μL) | (1.5 μL) | (3%) |
| ddH2O | Add to 20 μL | Add to 50 μL |
a: When using plasmid or phage DNA as template, the recommended dosage is 10ng-1pg/50 μL. When using genomic DNA as template, the recommended dosage is 250ng to 50 ng/50μL. When using cDNA as a template, it is recommended to dilute 2-100 times and add no more than 10% of the total system. Excessive templates can easily lead to non-specific amplification, and too few templates can easily lead to low PCR amplification efficiency;
b: The final primer concentration ranges from 0.2-1.0μM, and the recommended primer concentration is 0.4μM. Too few primers may lead to low yield or no amplification, and too many primers may lead to non-specific amplification;
c: High GC templates can add an additional DMSO up to 10% of the total volume to the reaction system.
2.Recommended PCR amplification conditions:
| Step | Temperature | Volume | Cycles |
| Initial Denaturationa | 95℃ | 30-120 s | 1 |
| Denaturationb | 95℃ | 5-10 s |
25-35 |
| Annealingc | 50-72℃ | 10-30 s | |
| Extensiond | 72℃ | 5-15 s/kb | |
| Final extension | 72℃ | 5-10 min | 1 |
| Hold | 4-16℃ | Forever |
a: The pre-denaturation time of 30 s is suitable for most conventional templates, and the denaturation time of complex templates can be extended to 2 min;
b: The short denaturation time is favorable for the amplification of long fragments over 20 KB;
c: For complex templates, the annealing time can be extended to 30 s for amplification with annexed primers. When the annealing temperature is between 68 ℃ and 70℃, the amplification specificity can be significantly improved by combining the annealing/extension temperature into annealing temperature;
d: Simple templates such as plasmids recommend an extension speed of 5-10s/kb; The recommended elongation rate for conventional genomic templates is 10-15s/kb, and complex template 15-30s/kb.
Note:
1.For different types of templates, the number of templates needs to be appropriate and adjusted according to the actual amplification situation.
2.The number of dNTPs and primers can be adjusted for amplification of fragments over 15 KB, and the designed length of primers is between 25 bp and 35 bp. It is recommended to use kit to extract high-quality templates.
3.Different PCR apparatus and PCR reaction tube will also affect the amplification of long fragment.
4.Primer design should follow primer design principles.
5.For complex templates, it is necessary to adjust the annealing temperature and extend the extension time to achieve efficient amplification.
6.Thoroughly thaw and mix before using. After complete melting, it can be stably stored at 4℃ for at least 2 weeks, avoiding repeated freeze-thaw.
7.Please wear disposable gloves and lab coat during operation.
For Research Use Only!
Ver. No.: V1.0-202102
|
Cat.No.
|
Product Name
|
Spec.
|
Operation
|
|---|
|
G3026-01
|
dNTP Mixture (10 mM each)
|
1 mL
|
|
|
G3026-05
|
dNTP Mixture (10 mM each)
|
5×1 mL
|
|
|
G3027-01
|
dNTP Mixture (2.5 mM each)
|
1 mL
|
|
|
G3027-05
|
dNTP Mixture (2.5 mM each)
|
5×1 mL
|
|
|
G3302-01
|
2 x Taq PCR Master Mix
|
1 mL
|
|
|
G3302-05
|
2 x Taq PCR Master Mix
|
5×1 mL
|
|
|
G3304-01
|
2 x Fast sTaq PCR Master Mix
|
1 mL
|
|
|
G3304-05
|
2 x Fast sTaq PCR Master Mix
|
5×1 mL
|
|
|
G3305-01
|
2 x Fast Pfus PCR Master Mix
|
1 mL
|
|
|
G3305-05
|
2 x Fast Pfus PCR Master Mix
|
5×1 mL
|
|
|
G3306-01
|
2 x Fast High Fidelity PCR Master Mix
|
1 mL
|
|
|
G3306-05
|
2 x Fast High Fidelity PCR Master Mix
|
5×1 mL
|
|
|
G3307-01
|
2 x LA PCR Master Mix
|
1 mL
|
|
|
G3307-05
|
2 x LA PCR Master Mix
|
5×1 mL
|
|
|
G3310-100T
|
Animal Tissue Direct PCR Kit
|
100 T
|
|
|
G3310-500T
|
Animal Tissue Direct PCR Kit
|
500 T
|
|
|
G3311-100T
|
Mouse Genotyping Kit
|
100 T
|
|
|
G3311-500T
|
Mouse Genotyping Kit
|
500 T
|