Description
Description
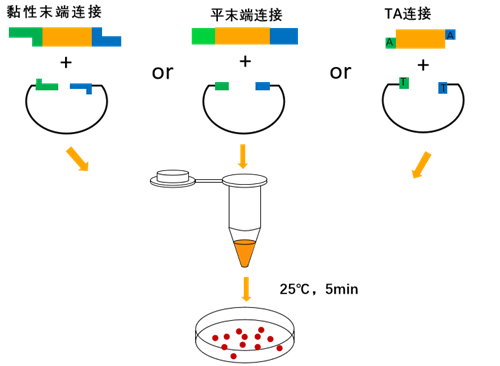
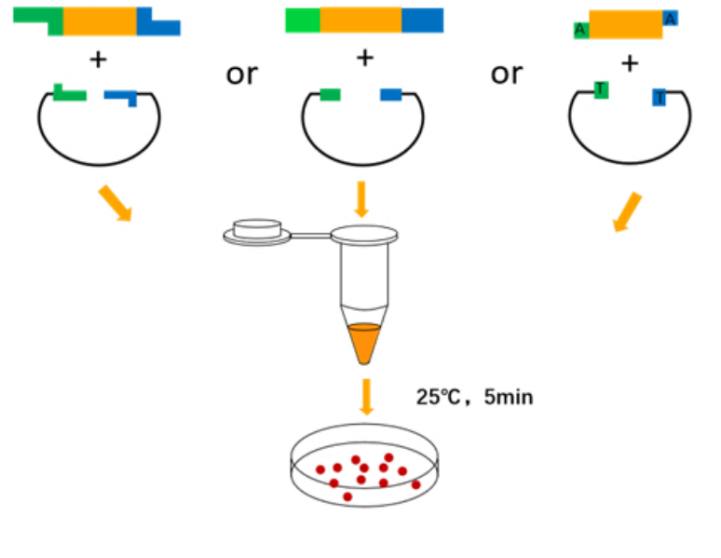
T4 DNA Ligase catalyzes the binding of phosphodiester bonds between the 5′-P and 3′-OH ends of sticky-end or blunt-ended double-stranded DNA or RNA. At the same time, the enzyme can also repair single-stranded nicks in double-stranded DNA, RNA, and DNA/RNA hybrid strands.
Definition of activity: One unit of enzyme can catalyze 1 nmol of 32P-labeled pyrophosphate into ATP by displacement reaction in 20 minutes at 37°C (one unit equals approximately 200 sticky end-linking units).
T4 DNA Ligase Storage buffer:20 mM Tris-HCl (pH 7.5), 50 mM KCl, 1 mM DTT, 50% (v/v) glycerol.
5×T4 DNA Ligase Buffer:250 mM Tris-HCl (pH 7.6), 50 mM MgCl2, 5 mM ATP, 5 mM DTT, Enhancer.
Storage and Handling Conditions
Transported with wet ice; stored at -20°C, valid for 12 months.
Component
| Component Number | Component | G3340-50 | G3340-100 |
| G3340-1 | T4 DNA Ligase | 250 U (50 μL) | 500 U (100 μL) |
| G3340-2 | 5×T4 DNA Ligase Buffer | 1 mL | 2×1 mL |
| Product Manual | |||
Assay Protocol
Ligation system (recommended 10 μL reaction system)
| Component | Volume |
| 5×T4 DNA Ligase Buffer | 2 μL |
| T4 DNA Ligase | 0.5-1 μL |
| Vector | X μL |
| DNA segment | Y μL |
| Nuclease-Free Water | Add to 10 μL |
Ligation reaction conditions
Sticky end ligation reaction at 25°C for 5-30 minutes; blunt end ligation reaction at 25°C does not exceed 2 hours or 4°C overnight.
Ligation product conversion
1. Take out the competent cells (such as E.coli DH5α, E.coli Top10, etc.) from the -80℃ refrigerator and place them on ice to thaw;
2. Add the reacted sample to the competent state, gently stir the bottom of the tube with fingers to mix, and place it in ice for 30 minutes;
3. The product was then placed in a 42°C water bath for heat shock for 90 s, and after that, it was quickly placed in ice for 2-5 min;
4. Take 900 μL of sterile SOC or LB medium and add it to the EP tube. After mixing, place the EP tube on a shaker and incubate at 220 rpm at 37°C for 1 h to recover the bacteria (it can also be cultured in a 37°C incubator for 1 h);
5. According to the experimental requirements, pipette different volumes of transformed competent cells and add them to the LB solid medium containing the corresponding antibiotics, spread the cells evenly, and after the liquid is completely absorbed, place the plate upside down in a 37°C incubator and cultivate overnight.
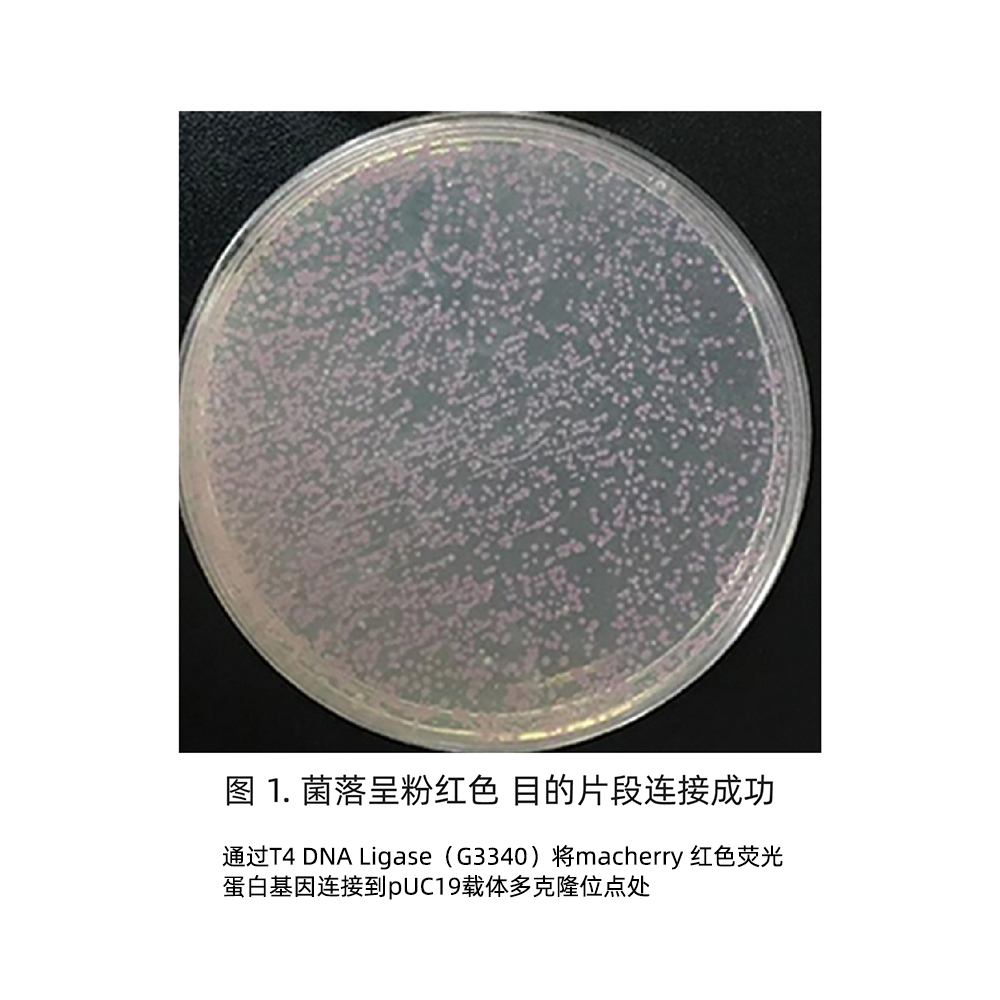
Positive clone identification
Pick the monoclonal colonies grown on the plate for colony PCR identification, or extract plasmids after culturing for digestion or PCR identification, or directly sequence and analyze the extracted plasmids for identification.
Note:
1. It is recommended that the reaction system be prepared on ice.
2. When the concentration of the carrier and the target fragment is low, it is not necessary to add water, and the carrier and the target fragment are directly used to supplement the system.
3. The recommended molar ratio of vector DNA to insert DNA fragment is 1:3~1:10.
4. It is normal for 5×T4 DNA Ligase Buffer to have a small amount of precipitation. It can be dissolved at 37°C and be mixed well before the experiment. It is recommended to freeze and store in aliquots after dissolving.
5. It is recommended to take out T4 DNA Ligase when it is used, and put it back to -20℃ immediately after use.
6. When electroporation is used for transformation, the ligation product needs to be purified by column method or ethanol precipitation method.
7. When the blunt-end vector is ligated with DNA fragments, the vector needs to be dephosphorylated (recommended G3400) to prevent its self-circularization.
8. Please wear lab coat and disposable gloves during operation.
For Research Use Only!
Ver. No.: V1.0-202111
|
Cat.No.
|
Product Name
|
Spec.
|
Operation
|
|---|
|
G3001-500ML
|
50×TAE Electrophoresis Buffer Solution
|
500 mL
|
|
|
G3002-250ML
|
10×TBE Electrophoresis Buffer Solution
|
250 mL
|
|
|
G3003-250ML
|
Servicebio® 10×TE(pH 8.0)
|
250 mL
|
|
|
G3004-100ML
|
DEPC Water
|
100 mL
|
|
|
G3013-100ML
|
RNA Extraction Solution
|
100 mL
|
|
|
G3320-05
|
2 × SYBR Green qPCR Master Mix (None ROX)
|
5 mL
|
|
|
G3321-05
|
2×SYBR Green qPCR Master Mix (Low ROX)
|
5 mL
|
|
|
G3322-05
|
2 × SYBR Green qPCR Master Mix (High ROX)
|
5 mL
|
|
|
G3323-05
|
2 × Fast SYBR Green qPCR Master Mix (None ROX)
|
5 mL
|
|
|
G3324-05
|
2 × Fast SYBR Green qPCR Master Mix (Low ROX)
|
5 mL
|
|
|
G3325-05
|
2 × Fast SYBR Green qPCR Master Mix (High ROX)
|
5 mL
|
|
|
G3330-100
|
SweScript RT I First Strand cDNA Synthesis Kit
|
100 rxns
|
|
|
G3340-100
|
T4 DNA Ligase (5 U/μL )
|
500 U
|
|
|
G5042-1G
|
Isopropyl β-D-1-thiogalactopyranoside (IPTG)
|
1 g
|
|
|
G5056-5G
|
Agarose(Electrophoresis-Grade, Low EEO)
|
5 g
|