Description
Please click to see video about the product. I
Product Information
| Product Name | Cat.No | Spec. |
| Annexin V-PE/7-AAD Cell Apoptosis Detection Kit | G1512-50T | 50 T |
| G1512-100T | 100 T |
Introduction
Apoptosis is a normal physiological process that occurs during embryonic development and maintenance of tissue homeostasis. The process of apoptosis is accompanied by many morphological changes, among which the loss of cell membrane is one of the early characteristics of apoptosis. In normal cells, phosphatidylserine (PS) is only distributed on the inner side of the phospholi7-ADDd bilayer of the cell membrane. However, in the early stage of apoptosis, PS will flip from the inner side of the li7-ADDd membrane to the outer side, exposing it to the outside of the cell. Annexin V is a Ca2+-dependent phospholi7-ADDd-binding protein with high affinity for PS and it can specifically bind to cells exposed to PS. Therefore, Annexin V is used as one of the indicators to detect early apoptosis of cells. Pro7-ADDdium iodide (7-ADD) is a nucleic acid dye that cannot penetrate normal cells with intact cell membranes and early apoptotic cells, but it can penetrate the cell membranes of late apoptotic and necrotic cells and stain cell nuclei.
This product uses a fusion protein composed of PE (Phycoerythrin) and Annexin V as a detection probe to detect early apoptosis of cells. At the same time, 7-AAD was used to distinguish surviving cells from necrotic and late apoptotic cells. Due to the combined use of Annexin V-PE and 7-ADD, live cells showed negative staining (Annexin V-/7-ADD-), early apoptotic cells showed single fluorescence positive (Annexin V+/7-ADD-), and late apoptotic cells and necrotic cells showed double fluorescence Fluorescence positive (Annexin V+/7-ADD+). This kit is suitable for flow cytometry or fluorescence microscopy. At the same time, since PE and Annexin V are 1:1 fusion, this product can also quantitatively detect apoptotic cells.
Storage and Handling Conditions
Transport with wet ice; store at 2-8℃ away from light, valid for 12 months.
Components
| Component Number | Component | G1512-50T | G1512-100T |
| G1512-1 | Annexin V-PE | 250 μL | 2×250 μL |
| G1512-2 | 7-AAD | 250 μL | 2×250 μL |
| G1512-3 | 1×Binding Buffer | 25 mL | 2×25 mL |
| Instruction Manual | 1 pc | ||
Experimental Procedure
1. Suspension of cells: Take the cell suspension and collect the cells by centrifugation at 500 g for 5 min at 4℃;
Adherent cells: Collect cell culture supernatant first. After digestion with trypsin without EDTA (recommended G4002), it was combined with the cell culture supernatant, and the cells were collected by centrifugation at 500 g for 5 min at 4℃. Trypsin digestion time should not be too long to avoid false positives caused by excessive digestion.
2. Wash the cells twice with pre-cooled PBS (recommended G4202), and collect the cells by centrifugation at 500 g for 5 min at 4℃ each time;
3. Gently resuspend the cells with pre-cooled 1×Binding Buffer, and adjust the cell concentration to 1~5×106/mL;
4. Take 100 μL of cell suspension, add 5 μL Annexin V-PE and 5 μL 7-ADD, mix them gently, and protect from light at room temperature for 8-10 min;
5. Add 400 µL of pre-chilled 1×Binding Buffer, shake gently, and use flow cytometry or fluorescence microscope for detection within 1 hour.
Result Analysis
1. Flow Cytometry Detection
a. Select the appropriate voltage and adjust the light compensation during the flow cytometer detection and analysis. Except for the experimental group, it is recommended to set a negative control (without Annexin V-PE and 7-ADD labeling) to adjust the voltage, and the single standard control (with Annexin V-EGFP only cells as well as PI only cells) were used to regulate compensation;
b. Reference example of flow cytometry detection and analysis:
Jurkat T lymphoma cells were induced with 5 µM Camptothecin for 6 h. Referring to the above experimental steps, flow cytometry was used for detection. The results are shown in the following figure.
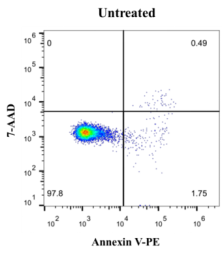
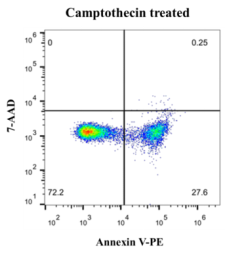
The maximum excitation wavelength of PE is 565 nm, and the maximum emission wavelength is 578 nm. The excitation maximum wavelength of the 7-ADD-DNA complex is 546 nm and the emission maximum wavelength is 647 nm. The two-chromatic scatter diagram was drawn by flow cytometry correlation analysis software, PE was located on the abscissa, and 7-ADD was located on the ordinate. In a ty7-ADDcal experiment, live cells are non-fluorescent and the scatter is located in the lower left first quadrant. The apoptotic cells in the early stage have strong green fluorescence, and the scattered points are located in the second quadrant of the lower right. Late apoptotic cells and necrotic cells showed double fluorescence in red and green, and the scattered points were located in the upper right third quadrant.
2. Fluorescence Microscopy Detection
a. Add 5-10 µL of Annexin V-PE and 7-ADD double-stained cell suspension to the slide.
b. Cover with a coverslip.
c. Observe with a two-color filter under a fluorescence microscope. Annexin V-PE has a green fluorescence signal, and 7-ADD has a red fluorescence signal.
Note
1. The entire experimental process should be handled as gently as possible to avoid cell breakage, which may affect the experimental results.
2. Washing the cells with PBS cannot be omitted, and the residual PBS should also be removed as much as possible.
3. When using trypsin to digest cells, the experiment should be handled carefully and the digestion time should be controlled to avoid artificial damage to the cells. If the digestion time is too short, the cells need to be vigorously beaten to fall off, which may easily cause mechanical damage to the cell membrane; if the digestion time is too long, the cell membrane is also easily damaged. affect the test results. In addition, trypsin containing EDTA cannot be used. EDTA will affect the binding of Annexin V to PS.
4. After the adherent cells are stimulated by apoptosis, if some cells float, it is necessary to collect the cell culture supernatant and the adherent cells together for staining, which will make the results more accurate.
5. Annexin V-PE and 7-ADD are sensitive to light, please avoid light during operation. Testing should be performed as soon as possible after the reaction is complete.
6. Please wear a lab coat and disposable gloves during the experiment to avoid pollution and ensure safety.
For Research Use Only!
|
Cat.No.
|
Product Name
|
Spec.
|
Operation
|
|---|
|
G1501-100T
|
Fluorescein (FITC) Tunel Cell Apoptosis Detection Kit
|
100 T
|
|
|
G1501-50T
|
Fluorescein (FITC) Tunel Cell Apoptosis Detection Kit
|
50 T
|
|
|
G1502-100T
|
TMR (Red) Tunel Cell Apoptosis Detection Kit
|
100 T
|
|
|
G1502-50T
|
TMR (Red) Tunel Cell Apoptosis Detection Kit
|
50 T
|
|
|
G1504-100T
|
CF488 Tunel Cell Apoptosis Detection Kit
|
100 T
|
|
|
G1504-50T
|
CF488 Tunel Cell Apoptosis Detection Kit
|
50 T
|
|
|
G1505-100T
|
CF640 Tunel Cell Apoptosis Detection Kit
|
100 T
|
|
|
G1505-50T
|
CF640 Tunel Cell Apoptosis Detection Kit
|
50 T
|
|
|
G1507-100T
|
DAB (SA-HRP) Tunel Cell Apoptosis Detection Kit
|
100 T
|
|
|
G1507-50T
|
DAB (SA-HRP) Tunel Cell Apoptosis Detection Kit
|
50 T
|
|
|
G1510-100T
|
Annexin V-EGFP/PI Cell Apoptosis Detection Kit
|
100 T
|
|
|
G1510-50T
|
Annexin V-EGFP/PI Cell Apoptosis Detection Kit
|
50 T
|
|
|
G1511-100T
|
Annexin V-FITC/PI Cell Apoptosis Detection Kit
|
100 T
|
|
|
G1511-50T
|
Annexin V-FITC/PI Cell Apoptosis Detection Kit
|
50 T
|
|
|
G1512-100T
|
Annexin V-PE/7-AAD Cell Apoptosis Detection Kit
|
100 T
|
|
|
G1512-50T
|
Annexin V-PE/7-AAD Cell Apoptosis Detection Kit
|
50 T
|
|
|
G1513-100T
|
Annexin V-IF488/PI Cell Apoptosis Detection Kit
|
100 T
|
|
|
G1513-50T
|
Annexin V-IF488/PI Cell Apoptosis Detection Kit
|
50 T
|
|
|
G1514-100T
|
Annexin V-IF647/PI Cell Apoptosis Detection Kit
|
100 T
|
|
|
G1514-50T
|
Annexin V-IF647/PI Cell Apoptosis Detection Kit
|
50 T
|
|
|
G1700-50T
|
Cell Cycle and Apoptosis Detection Kit
|
50 T
|