Description
Product Information
| Product Name | Cat.No | Spec. |
| Dil Cell Membrane Red Fluorescence Staining Kit | G1705 | 100-1000 T |
Description
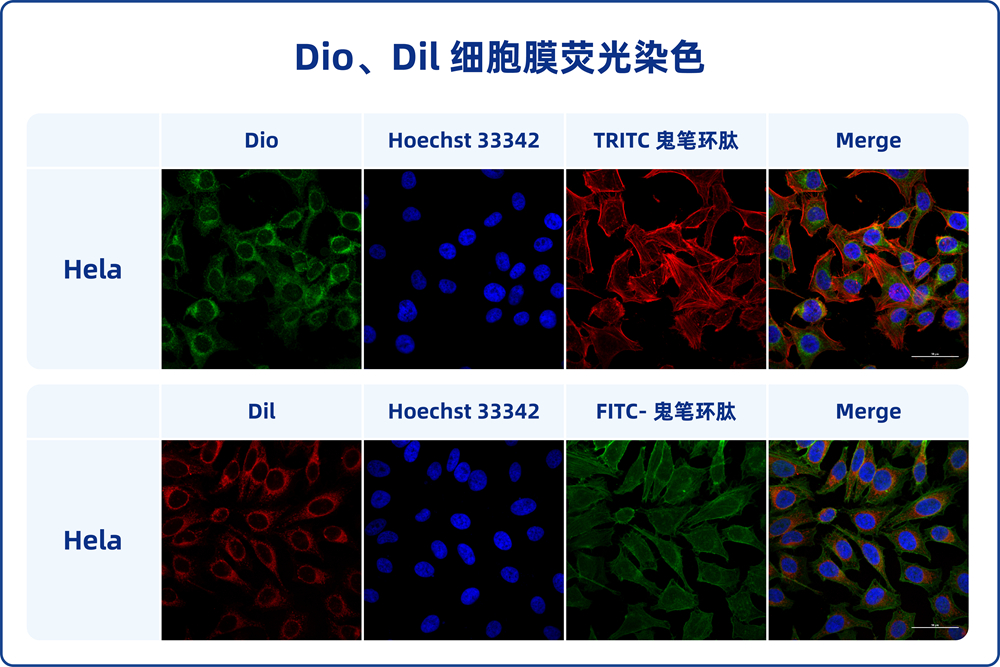
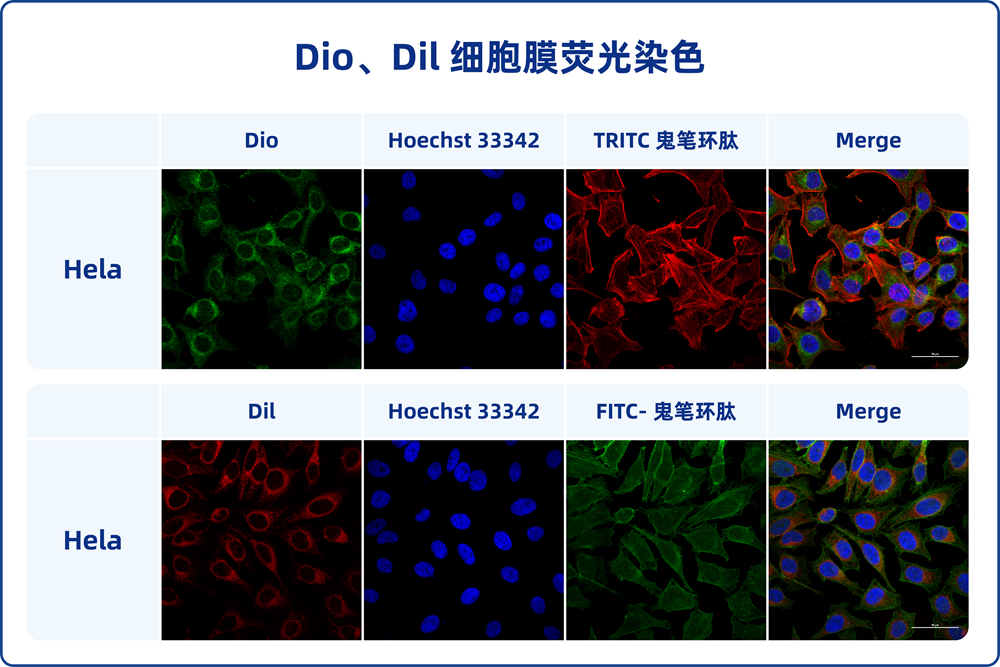
DiI, which is also known as DiIC18(3), 1,1′-dioctagyl-3,3′,3′-tetramethyl indole carbonyl cyanine perchlorate, molecular weight 933.87, is a kind of long chain dialkyl carbocyanine dye fluorescent dye with lipophilic property. It is often used to label cell membranes and other lipid-soluble biological structures. After entering the cell membrane, Dil can spread laterally and gradually stain the entire cell membrane. Dil fluorescence is very weak before entering the cell membrane, but when combined with the cell, the fluorescence intensity will be greatly enhanced. After being excited, Dil can emit orange-red fluorescence, which can be detected by standard FITC filter. The maximum excitation wavelength of Dil is 550nm, and the maximum emission wavelength is 564nm. According to the characteristics of Dil, it can be used to stain living cells as well as fixed cells or tissues. In addition, Dil probes generally do not affect the viability of cells, so forward or reverse labeled cells or some substances (exosomes) can be used as tracer detection.
This product Dil cell membrane green fluorescence staining kit contains Dil fluorescence probe and optimized staining buffer, which can make cell membrane staining faster, fluorescence brighter and more stable, and better effect.
Storage and Handling Conditions
Transport with wet ice; Store at -20°C in the dark, avoid repeated freezing and thawing of probes, valid for 6 months.
Component
| Component Number | Component | G1704 |
| G1705-1 | Dil Cell Membrane Red Fluorescent Probe | 100μL |
| G1705-2 | Staining Buffer | 100mL |
| Manuals | One copy | |
Assay Protocol / Procedures
1. Preparation of Dil staining working solution:
1.1. Dil staining working solution (prepared when using) was prepared by mixing and diluting Dil cell membrane Red fluorescent probe with staining buffer at a ratio of 1:1000; Note that the Dil probe dilution ratio can be adjusted from 1:500 to 1:2000 according to the specific situation to obtain the best staining effect.
2. Staining of suspension of live cell:
2.1. The suspended cells were collected and centrifuged at 500-1000 g at room temperature for 3-5 min to remove the cell supernatant;
2.2. Cells were resuspended with Dil staining working solution to make the cell density at 1-2×106 cells /mL;
2.3. Incubate at 37℃ for 5-20 min in the dark; It is recommended to adjust the staining time according to the specific cells to obtain the best staining effect.
2.4. At the end of incubation, centrifuge 500-1000 g at room temperature for 3-5 min to aspirate Dil staining working solution;
2.5. Resuspended cells with 37℃ preheated PBS or cell medium, centrifuged at 500-1000 g for 3-5 min, and removed supernatant;
2.6. Repeat Step 2.5 once;
2.7. The cells were detected by flow cytometry, or transferred to porous plates, cell culture dishes or slides, and observed under fluorescence microscopy. The maximum excitation wavelength of Dil is 550nm, and the maximum emission wavelength is 564nm.
3. Staining of adherent live cells (6-well plate as an example):
3.1. The cells were planted in 6-well plates at a certain density in advance;
3.2. The cell medium was removed by aspiration, and the cells were washed twice with PBS (G4202). 1 mL Dil staining working solution was added (well plates with other specifications were adjusted according to the situation to ensure dye coverage of cells);
3.3. Incubate at 37℃ for 5-20 min in the dark, and remove Dil staining working solution by suction. It is suggested to adjust the staining time according to the specific cells to obtain the best staining effect.
3.4. Wash the cells 1-2 times with 37℃ preheated PBS or cell medium;
3.5. Add PBS preheated at 37℃ or cell medium and observe under fluorescence microscope. The maximum excitation wavelength of Dil is 550nm, and the maximum emission wavelength is 564nm.
4. Staining of fixed adherent cells or sections:
4.1. Sample pretreatment:
For cells: Remove the cell medium and wash 1-2 times with PBS. 4% paraformaldehyde fixing solution (G1101) was added and fixed for 10 min at room temperature. Remove fixation solution by suction and wash with PBS 2-3 times.
For tissue sections: paraffin sections are dewaxed; Frozen sections were rewarmed and washed with water.
4.2. Cells or sections were treated by adding 0.1-0.5% Triton-100 (prepared with PBS) and permeated for 10 min at room temperature; The permeable liquid was removed and washed with PBS 2-3 times;
4.3. (Optional, immunofluorescent labeling) Incubation of antibodies or staining with other dyes according to immunofluorescent staining method. Note: In the process of antibody incubation, the blocking solution, antibody diluent, washing solution and so on should not contain scaling agent.
4.4. Add an appropriate volume of Dil staining working solution to cover cells or tissues, incubate at 37°C for 5-20 min in the dark, and remove Dil staining working solution. It is recommended to adjust the staining time according to the specific cell or tissue samples to obtain the best staining effect.
4.5. Cells or sections were washed with PBS for 2-3 times and then placed under a fluorescence microscope (cells should be covered with appropriate amount of PBS). The maximum excitation wavelength of Dil is 550nm, and the maximum emission wavelength is 564nm.
5. Fluorescent labeling of exosomes:
5.1. The extracted exosomes were precipitated and resuspended with an appropriate amount of Dil staining working solution;
5.2. Incubate the sample at 37℃ for 30 min in the dark;
5.3. Dilute the sample volume with 10 times the volume of PBS (optional);
5.4. Exosomes were extracted again according to the previous extraction protocol to remove excess dyes;
5.5. The collected exosomes were precipitated and resuspended in PBS to obtain DIl-labeled exosomes. It can be used for subsequent experiments, such as cellular uptake.
Note
1. All fluorescent dyes have quenching problems, please pay attention to avoid light to slow down fluorescence quenching.
2. Due to the lipophilic characteristics of the probe, please avoid using reagents containing glycerol or other organic matter during the experiment, otherwise the staining effect will be affected.
3. When fixing operation is required, the sample should be fixed in 4% paraformaldehyde, because other inappropriate fixing solutions will lead to high fluorescence background.
4. The optimum dilution ratio and incubation time of the probe should be adjusted according to the actual situation due to the different sensitivity between cells and experimental purposes.
5. For your health and safety, please wear laboratory coat and gloves when operating.
For Research Use Only!
|
Cat.No.
|
Product Name
|
Spec.
|
Operation
|
|---|
|
G1011-10ML
|
Hoechst 33258 Staining Solution (Ready-To-Use)
|
10 mL
|
|
|
G1012-100ML
|
DAPI Staining Reagent (Ready to Use)
|
100 mL
|
|
|
G1012-10ML
|
DAPI Staining Reagent (Ready to Use)
|
10 mL
|
|
|
G1021-10ML
|
PI Stain
|
10 mL
|
|
|
G1127-1ML
|
Hoechst 33342 Staining Solution
|
1 mL
|
|
|
G1515-100T
|
JC-1 Mitochondrial Membrane Potential Assay Kit
|
100 T
|
|
|
G1704
|
DiO Cell Membrane Green Fluorescence Staining Kit
|
100-1000 T
|
|
|
G1705
|
Dil Cell Membrane Red Fluorescence Staining Kit
|
100-1000
|