Description
Product Information
Product Name: Protein Immunoprecipitation Kit (Protein A Magnetic Bead Method)
Product Number: G2209-50T
Specifications: 50T
Product Introduction:
Immunoprecipitation or co-immunoprecipitation is a common technique used to study protein-protein interactions (PPIs). It involves the use of specific antibodies and media that can bind to these antibodies, such as Protein A/G Agarose or Protein A/G Magrose, or directly using media coupled with specific antibodies (such as agarose gel or magnetic beads). This technique allows the separation of antigen-antibody complexes from a solution through centrifugation or magnetic force, thereby achieving the separation and purification of the target protein. The purified protein can then be used for protein immunoblotting (Western Blot) or mass spectrometry analysis.
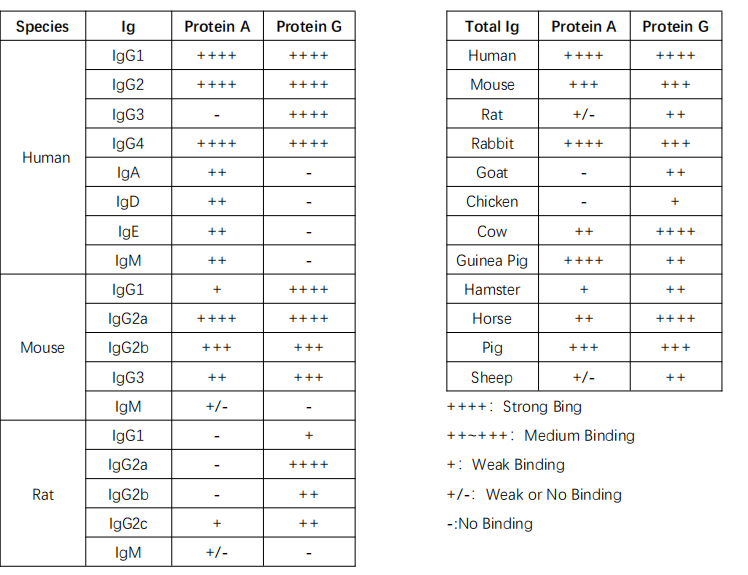
Protein A is a cell wall surface protein found in Staphylococcus aureus with a molecular weight of 42 kDa. Protein G, on the other hand, is an immunoglobulin-binding protein expressed by C-type or G-type Streptococcal bacteria. Both Protein A and Protein G function similarly in specifically binding to mammalian immunoglobulins (Ig).
Recombinant Protein A and G bound to magnetic beads can be used for immunoprecipitation or antibody purification. Protein A magnetic beads are suitable for immunoprecipitating human IgG1, IgG2, IgG4, mouse IgG2a, IgG2b, and others. Meanwhile, Protein G magnetic beads are suitable for immunoprecipitating human IgG1, IgG2, IgG3, IgG4, mouse IgG1, IgG2a, IgG2b, IgG3, rat IgG1, IgG2a, IgG2b, IgG2c, and other polyclonal antibodies (specific information can be found in Table 1).
This product uses Protein A-labeled magnetic beads developed in-house. Compared to similar products on the market, this product has a higher antibody binding efficiency and lower non-specific binding. When used with optimized buffers, it facilitates convenient and efficient immunoprecipitation experiments. It can be widely applied to the separation and purification of target proteins in cell lysates, cell culture supernatants, serum, ascites, and other samples. This kit provides two elution methods (denaturing elution and acidic elution) for the elution of target proteins. Acidic elution, in particular, does not contain antibody heavy and light chains after elution, effectively addressing the issue of interference from antibody heavy and light chains in protein immunoblotting experiments after immunoprecipitation.
| Product Information | |
|---|---|
| Features | Description |
| Product Content | 50 mg/ml magnetic beads stored in special preservation solution |
| Magnetic Type | Superparamagnetic |
| Coupling Protein | Protein A |
| Coupling Protein Molecular Weight | ~25 kDa (Protein A) |
| Binding Capacity | >1 mg mouse antibody per milliliter of beads |
| Specificity | Antibodies from various species, including mouse, human, rat, goat, sheep, and cow |
| Bead Size | 30~150 μm |
| Elution Methods | Acidic elution or elution with 1× protein sample buffer (reducing) |
| Note: | When eluted with 1× protein sample buffer (reducing), antibody heavy chain (~50 kDa) and light chain (~25 kDa) are denatured and released from the beads |
| Applications | Protein immunoprecipitation, co-immunoprecipitation, protein purification and separation |
| Storage and Transport | |
| Transport | Shipped with wet ice |
| Storage (1× protein sample buffer) | Store at -20°C |
| Other Storage | Store at 2-8°C |
| Shelf Life | 12 months |
| Component Number | Component | Quantity | Storage Temperature |
|---|---|---|---|
| G2209-50T | IP Lysis Buffer | 50 mL | 2-8℃ |
| G0015 | 10× TBS Buffer | 5 mL | 2-8℃ |
| G2209-1 | SweMagrose Protein A | 1 mL | 2-8℃ |
| G2209-2 | Acid Elution Buffer | 10 mL | 2-8℃ |
| G2209-3 | Neutralization Elution Buffer | 1 mL | 2-8℃ |
| G2013 | 5× Protein Sample Buffer (Reducing) | 1 mL | -20℃ |
| Instruction Manual | User Manual | 1 copy |
| Product Name | Cat. Number | Spec. |
|---|---|---|
| 50× Cocktail Protease Inhibitor | G2006-250UL | 250 μL |
| PBS, 1× (Phosphate Buffered Saline) | G4202-500ML | 500 mL |
These are the reagents that are not included in the kit and should be provided separately for use with the kit.
Step 1: Preparation of the Kit
a) Refer to the table below and prepare the relevant reagents according to the ratio of 100-500 μL of IP Lysis Buffer for each sample.
Here are the steps you’ve provided along with the required solutions and their volumes, presented in a table format:
| Step | Required Solution | Volume (100 μL) | Volume (500 μL) |
|---|---|---|---|
| Cell Lysis and Preparation | IP Lysis Buffer with added 50× Cocktail Protease Inhibitor | 100 μL | 500 μL |
| Immunoprecipitation | SweMagrose Protein A | 4 μL | 20 μL |
| Wash (3 times) | 1× TBS | 100 μL | 500 μL |
| Acid Elution and Neutralization | Acid Elution Buffer | 20 μL | 100 μL |
| Neutralization Buffer | 20 μL | 100 μL | |
| Denaturing Elution | 1× Protein Sample Buffer (Reducing) | 20 μL | 100 μL |
This table summarizes the steps, the required solutions, and their respective volumes for both 100 μL and 500 μL sample sizes.
Step 1: Preparation of the Kit
a) Preparation of IP Lysis Buffer with Protease Inhibitor:
- Prepare IP Lysis Buffer containing protease inhibitor according to the ratio of 100-500 μL IP Lysis Buffer per sample. For example, mix IP Lysis Buffer with 50× Cocktail Protease Inhibitor at a 50:1 ratio. For 1 mL IP Lysis Buffer, add 20 μL of 50× Cocktail Protease Inhibitor. Store the prepared IP Lysis Buffer with protease inhibitor in an ice bath or at 4°C.
b) Note: If the target protein in your immunoprecipitation involves phosphorylation or acetylation modifications, you may need to add phosphatase inhibitors or deacetylase inhibitors (not included in the kit) to your IP Lysis Buffer. It’s recommended to prepare the IP Lysis Buffer with inhibitors just before use, rather than preparing and freezing it for later use.
c) Preparation of 1× TBS:
- Dilute 10× TBS Buffer with ultrapure water to achieve a 1× concentration. For example, add 1 mL of 10× TBS Buffer to 9 mL of ultrapure water and mix well to obtain 1× TBS Buffer.
d) Preparation of 1× Protein Sample Buffer (Reducing):
- Take an appropriate amount of 5× Protein Sample Buffer (Reducing) and dilute it 5-fold with ultrapure water to make 1× Protein Sample Buffer (Reducing). For example, mix 0.2 mL of 5× Protein Sample Buffer (Reducing) with 0.8 mL of ultrapure water and mix well to obtain 1× Protein Sample Buffer (Reducing).
Step 2: Preparation of Antigen Samples
- Prepare your samples immediately after cell lysis or tissue preparation. If immediate use is not possible, store the samples at -20°C or -80°C, although freeze-thaw cycles may affect protein-protein interactions.
For Tissue Samples:
a) Take the appropriate amount of tissue and wash it with pre-chilled PBS (recommended G4202) to remove blood contaminants. Cut it into small pieces and place them in a homogenizer.
b) Add 100-200 μL of IP Lysis Buffer with protease inhibitor per 10-20 mg of tissue. If higher protein concentrations are needed, adjust the volume of IP Lysis Buffer accordingly.
c) Homogenize the tissue using a glass homogenizer or a hand-held homogenizer. Alternatively, use a low-temperature grinder such as KZ-III-F.
d) Transfer the homogenate to a 1.5 mL centrifuge tube, vortex to mix, incubate on ice for 30 minutes, and use a pipette to agitate every 10 minutes to ensure complete cell lysis.
e) Centrifuge at 12,000 g at 4°C for 5 minutes to collect the supernatant, which is the total protein solution for subsequent immunoprecipitation or co-immunoprecipitation experiments.
For Adherent Cell Samples:
a) If necessary, wash the cells with PBS 2-3 times, with the final wash removing all residual liquid.
b) Scrape the cells or trypsinize them to create a suspension. Collect the cells in a 1.5 mL centrifuge tube by centrifugation at 1000 g for 5 minutes at 4°C. Discard the supernatant to obtain the cell pellet.
c) Add 100-200 μL of IP Lysis Buffer with protease inhibitor per 50-100 million cells (equivalent to one well of a 6-well plate). Gently pipette or flick the tube to ensure complete cell suspension. Incubate on ice for 3-5 minutes until complete cell lysis is achieved. After complete lysis, there should be no visible cell pellet. If there are a significant number of cells, consider splitting them into 50-100 million cells/tube and then lyse to ensure thorough lysis.
d) After complete lysis, centrifuge at 12,000 g at 4°C for 5 minutes. Collect the supernatant, which is the total protein solution for subsequent immunoprecipitation or co-immunoprecipitation experiments.
For Suspension Cell Samples:
a) Collect the cell pellet by centrifugation at 1000 g for 5 minutes at 4°C. If necessary, wash the cells once with PBS and then remove any residual liquid.
b) Add 100-200 μL of IP Lysis Buffer with protease inhibitor per 50-100 million cells. Gently pipette to disperse the cells. If there are a large number of cells, it is recommended to split them into 50-100 million cells/tube and then lyse to ensure complete lysis.
c) Incubate on ice for 5 minutes. Then, centrifuge at 12,000 g at 4°C for 5 minutes and collect the supernatant, which is the total protein solution for subsequent immunoprecipitation or co-immunoprecipitation experiments.
For Bacterial or Yeast Samples:
a) Take 1 mL of bacterial or yeast culture and centrifuge to remove the supernatant. If necessary, wash once with PBS and remove any residual liquid. Gently vortex or tap the tube to ensure even dispersion of the cells.
b) Add 100-200 μL of IP Lysis Buffer with protease inhibitor to the cells. Gently pipette to disperse the cells thoroughly.
c) Incubate on ice for 10 minutes. If better lysis is desired,